Immunity ( IF 25.5 ) Pub Date : 2024-09-02 , DOI: 10.1016/j.immuni.2024.08.007 Konstantin Stark 1 , Badr Kilani 1 , Sven Stockhausen 1 , Johanna Busse 2 , Irene Schubert 2 , Thuy-Duong Tran 2 , Florian Gaertner 3 , Alexander Leunig 1 , Kami Pekayvaz 1 , Leo Nicolai 1 , Valeria Fumagalli 4 , Julia Stermann 1 , Felix Stephan 1 , Christian David 5 , Martin B Müller 6 , Birgitta Heyman 7 , Anja Lux 8 , Alexandra da Palma Guerreiro 9 , Lukas P Frenzel 9 , Christoph Q Schmidt 10 , Arthur Dopler 10 , Markus Moser 11 , Sue Chandraratne 2 , Marie-Luise von Brühl 2 , Michael Lorenz 2 , Thomas Korff 12 , Martina Rudelius 13 , Oliver Popp 14 , Marieluise Kirchner 14 , Philipp Mertins 14 , Falk Nimmerjahn 8 , Matteo Iannacone 4 , Markus Sperandio 5 , Bernd Engelmann 15 , Admar Verschoor 16 , Steffen Massberg 1
|
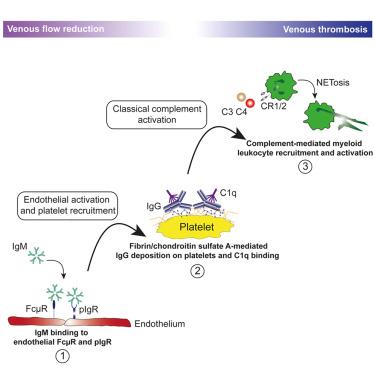
Venous thromboembolism (VTE) is a common, deadly disease with an increasing incidence despite preventive efforts. Clinical observations have associated elevated antibody concentrations or antibody-based therapies with thrombotic events. However, how antibodies contribute to thrombosis is unknown. Here, we show that reduced blood flow enabled immunoglobulin M (IgM) to bind to FcμR and the polymeric immunoglobulin receptor (pIgR), initiating endothelial activation and platelet recruitment. Subsequently, the procoagulant surface of activated platelets accommodated antigen- and FcγR-independent IgG deposition. This leads to classical complement activation, setting in motion a prothrombotic vicious circle. Key elements of this mechanism were present in humans in the setting of venous stasis as well as in the dysregulated immunothrombosis of COVID-19. This antibody-driven thrombosis can be prevented by pharmacologically targeting complement. Hence, our results uncover antibodies as previously unrecognized central regulators of thrombosis. These findings carry relevance for therapeutic application of antibodies and open innovative avenues to target thrombosis without compromising hemostasis.
中文翻译:

抗体和补体是血栓形成的关键驱动因素
静脉血栓栓塞 (VTE) 是一种常见的致命疾病,尽管采取了预防措施,但发病率仍在不断增加。临床观察发现抗体浓度升高或基于抗体的治疗与血栓事件相关。然而,抗体如何促进血栓形成尚不清楚。在这里,我们发现血流减少使得免疫球蛋白 M (IgM) 能够与 FcμR 和聚合免疫球蛋白受体 (pIgR) 结合,从而启动内皮激活和血小板募集。随后,活化血小板的促凝表面适应抗原和 FcγR 独立的 IgG 沉积。这导致经典的补体激活,引发血栓前恶性循环。这种机制的关键要素存在于人类静脉淤滞以及 COVID-19 免疫血栓失调的情况下。这种抗体驱动的血栓形成可以通过药物靶向补体来预防。因此,我们的结果揭示了抗体是以前未被认识的血栓形成的中央调节因子。这些发现与抗体的治疗应用相关,并为在不影响止血的情况下靶向血栓形成开辟了创新途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号