Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition metal-free difunctionalization of unactivated alkenes: Arylation/azidation, arylation/chlorination, and arylation/cyanation
Chem ( IF 19.1 ) Pub Date : 2024-09-02 , DOI: 10.1016/j.chempr.2024.07.036 Li Li , Viresh H. Rawal
Chem ( IF 19.1 ) Pub Date : 2024-09-02 , DOI: 10.1016/j.chempr.2024.07.036 Li Li , Viresh H. Rawal
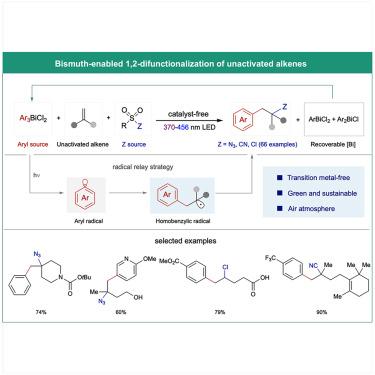
|
Arylethylamines represent a privileged scaffold in pharmaceutical compounds and form the backbone of many medical drugs, including those used for treating neurological diseases and pain. Their biomedical significance has inspired new synthetic methods that rely on transition metal-catalyzed aminoarylation reaction to an alkene, often in conjunction with a photoredox catalyst or a photosensitizer and guided by a directing or stabilizing group. Here, we introduce a simple and effective method for the azidoarylation of unactivated alkenes under transition metal-free conditions. Visible- or near-UV-light irradiation of readily available triarylbismuth dichlorides generates an aryl radical that selectively adds to the alkene, and the resulting homobenzyl radical is intercepted by an amine equivalent. This method offers a broad substrate scope and also enables the arylchlorination and arylcyanation of unactivated alkenes.
中文翻译:

未活化烯烃的无过渡金属二官能化:芳基化/叠氮化、芳基化/氯化和芳基化/氰化
芳乙胺是药物化合物中的特权支架,是许多医疗药物的主干,包括用于治疗神经系统疾病和疼痛的药物。它们的生物医学意义激发了新的合成方法,这些方法依赖于过渡金属催化的氨基芳基化反应到烯烃,通常与光氧化还原催化剂或光敏剂结合,并由定向或稳定基团引导。在这里,我们介绍了一种在无过渡金属条件下对未活化烯烃进行叠氮基化的简单有效的方法。对现成的三芳基二氯化物进行可见光或近紫外光照射会产生芳基,该芳基自由基选择性地添加到烯烃中,所得的同苄自由基被胺当量截留。该方法提供了广泛的底物范围,并且还能够对未活化的烯烃进行芳基氯化和芳氰化。
更新日期:2024-09-02
中文翻译:

未活化烯烃的无过渡金属二官能化:芳基化/叠氮化、芳基化/氯化和芳基化/氰化
芳乙胺是药物化合物中的特权支架,是许多医疗药物的主干,包括用于治疗神经系统疾病和疼痛的药物。它们的生物医学意义激发了新的合成方法,这些方法依赖于过渡金属催化的氨基芳基化反应到烯烃,通常与光氧化还原催化剂或光敏剂结合,并由定向或稳定基团引导。在这里,我们介绍了一种在无过渡金属条件下对未活化烯烃进行叠氮基化的简单有效的方法。对现成的三芳基二氯化物进行可见光或近紫外光照射会产生芳基,该芳基自由基选择性地添加到烯烃中,所得的同苄自由基被胺当量截留。该方法提供了广泛的底物范围,并且还能够对未活化的烯烃进行芳基氯化和芳氰化。































 京公网安备 11010802027423号
京公网安备 11010802027423号