当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Based Design and Development of Phosphine Oxides as a Novel Chemotype for Antibiotics that Dysregulate Bacterial ClpP Proteases
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-02 , DOI: 10.1021/acs.jmedchem.4c00773 Funing Lin 1 , Mark F Mabanglo 2 , Jin Lin Zhou 1 , Gursonika Binepal 3 , Marim M Barghash 2 , Keith S Wong 2 , Scott D Gray-Owen 3 , Robert A Batey 1, 4 , Walid A Houry 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-02 , DOI: 10.1021/acs.jmedchem.4c00773 Funing Lin 1 , Mark F Mabanglo 2 , Jin Lin Zhou 1 , Gursonika Binepal 3 , Marim M Barghash 2 , Keith S Wong 2 , Scott D Gray-Owen 3 , Robert A Batey 1, 4 , Walid A Houry 1, 2
Affiliation
|
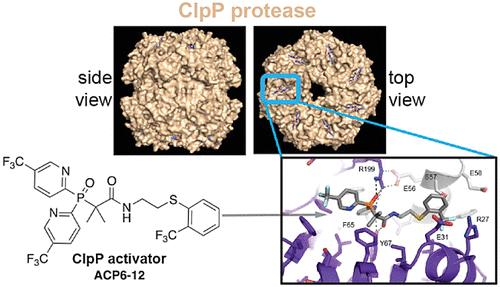
A series of arylsulfones and heteroarylsulfones have previously been demonstrated to dysregulate the conserved bacterial ClpP protease, causing the unspecific degradation of essential cellular housekeeping proteins and ultimately resulting in cell death. A cocrystal structure of a 2-β-sulfonylamide analog, ACP1-06, with Escherichia coli ClpP showed that its 2-pyridyl sulfonyl substituent adopts two orientations in the binding site related through a sulfone bond rotation. From this, a new bis-aryl phosphine oxide scaffold, designated as ACP6, was designed based on a “conformation merging” approach of the dual orientation of the ACP1-06 sulfone. One analog, ACP6-12, exhibited over a 10-fold increase in activity over the parent ACP1-06 compound, and a cocrystal X-ray structure with ClpP confirmed its predicted binding conformation. This allowed for a comparative analysis of how different ligand classes bind to the hydrophobic binding site. The study highlights the successful application of structure-based rational design of novel phosphine oxide-based antibiotics.
中文翻译:

基于结构的氧化膦的设计和开发作为抗生素的新型化学型,可调节细菌 ClpP 蛋白酶
一系列芳基砜和杂芳基砜先前已被证明可以使保守的细菌 ClpP 蛋白酶失调,导致重要的细胞管家蛋白的非特异性降解,并最终导致细胞死亡。 2-β-磺酰胺类似物 ACP1-06 与大肠杆菌ClpP 的共晶结构表明,其 2-吡啶磺酰基取代基在通过砜键旋转相关的结合位点中采用两个方向。由此,基于ACP1-06砜双重取向的“构象合并”方法,设计了一种新的双芳基氧化膦支架,命名为ACP6。一种类似物 ACP6-12 的活性比母体 ACP1-06 化合物增加了 10 倍以上,并且与 ClpP 的共晶 X 射线结构证实了其预测的结合构象。这允许对不同配体类别如何与疏水结合位点结合进行比较分析。该研究强调了基于结构的合理设计新型氧化膦类抗生素的成功应用。
更新日期:2024-09-02
中文翻译:

基于结构的氧化膦的设计和开发作为抗生素的新型化学型,可调节细菌 ClpP 蛋白酶
一系列芳基砜和杂芳基砜先前已被证明可以使保守的细菌 ClpP 蛋白酶失调,导致重要的细胞管家蛋白的非特异性降解,并最终导致细胞死亡。 2-β-磺酰胺类似物 ACP1-06 与大肠杆菌ClpP 的共晶结构表明,其 2-吡啶磺酰基取代基在通过砜键旋转相关的结合位点中采用两个方向。由此,基于ACP1-06砜双重取向的“构象合并”方法,设计了一种新的双芳基氧化膦支架,命名为ACP6。一种类似物 ACP6-12 的活性比母体 ACP1-06 化合物增加了 10 倍以上,并且与 ClpP 的共晶 X 射线结构证实了其预测的结合构象。这允许对不同配体类别如何与疏水结合位点结合进行比较分析。该研究强调了基于结构的合理设计新型氧化膦类抗生素的成功应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号