当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design Glutamate Dehydrogenase for Nonaqueous System by Motifs Reassembly and Interaction Network Analysis
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-09-02 , DOI: 10.1021/acs.jafc.4c02995 Qian Zhang 1 , Yuxin Chen 1 , Lingxuan Duan 1 , Lingling Dong 1 , Shizhen Wang 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-09-02 , DOI: 10.1021/acs.jafc.4c02995 Qian Zhang 1 , Yuxin Chen 1 , Lingxuan Duan 1 , Lingling Dong 1 , Shizhen Wang 1, 2
Affiliation
|
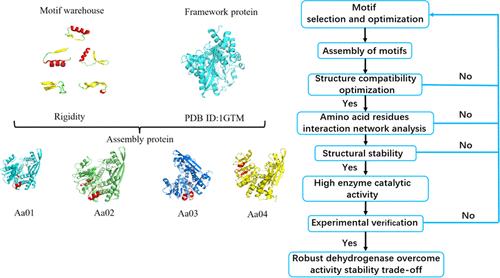
Glutamate dehydrogenases (GDH) serve as the key regulated enzyme that links protein and carbohydrate metabolism. Combined with motif reassembly and mutation, novel GDHs were designed. Motif reassembly of thermophilic GDH and malate dehydrogenase aims to overcome stability and activity tradeoff in nonaqueous systems. Structural compatibility and dynamic cooperation of the designed AaDHs were studied by molecular dynamics simulation. Furthermore, multipoint mutations improved its catalytic activity for unnatural substrates. Amino acid interaction network analysis indicated that the high density of hydrogen-bonded salt bridges is beneficial to the stability. Finally, the experimental verification determines the kinetics of AaDHs in a nonaqueous system. The activity of Aa05 was increased by 1.78-fold with ionic liquid [EMIM]BF4. This study presents the strategy of a combination of rigid motif assembly and mutations of active sites for robust dehydrogenases with high activity in the nonaqueous system, which overcomes the activity–stability tradeoff effect.
中文翻译:

通过基序重组和相互作用网络分析为非水体系设计谷氨酸脱氢酶
谷氨酸脱氢酶 (GDH) 是连接蛋白质和碳水化合物代谢的关键调节酶。结合基序重组和突变,设计了新的GDHs。嗜热 GDH 和苹果酸脱氢酶的基序重组旨在克服非水系统中的稳定性和活性权衡。通过分子动力学模拟研究了所设计的 AaDHs 的结构相容性和动态合作。此外,多点突变提高了其对非天然底物的催化活性。氨基酸相互作用网络分析表明,氢键盐桥的高密度有利于稳定性。最后,实验验证确定了 AaDHs 在非水系统中的动力学。离子液体 [EMIM]BF4 使 Aa05 的活性增加了 1.78 倍。本研究提出了刚性基序组装和活性位点突变相结合的策略,以获得在非水系统中具有高活性的稳健脱氢酶,从而克服了活性-稳定性权衡效应。
更新日期:2024-09-02
中文翻译:

通过基序重组和相互作用网络分析为非水体系设计谷氨酸脱氢酶
谷氨酸脱氢酶 (GDH) 是连接蛋白质和碳水化合物代谢的关键调节酶。结合基序重组和突变,设计了新的GDHs。嗜热 GDH 和苹果酸脱氢酶的基序重组旨在克服非水系统中的稳定性和活性权衡。通过分子动力学模拟研究了所设计的 AaDHs 的结构相容性和动态合作。此外,多点突变提高了其对非天然底物的催化活性。氨基酸相互作用网络分析表明,氢键盐桥的高密度有利于稳定性。最后,实验验证确定了 AaDHs 在非水系统中的动力学。离子液体 [EMIM]BF4 使 Aa05 的活性增加了 1.78 倍。本研究提出了刚性基序组装和活性位点突变相结合的策略,以获得在非水系统中具有高活性的稳健脱氢酶,从而克服了活性-稳定性权衡效应。































 京公网安备 11010802027423号
京公网安备 11010802027423号