当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing Real Active Sites in Intricate Grain Boundaries Assemblies on Electroreduction of CO2 to C2+ Products
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2024-09-02 , DOI: 10.1002/aenm.202402636
Lei Wang 1 , Xue Yao 2 , Holly Fruehwald 3 , Dmitry Akhmetzyanov 3 , Mathew Hanson 1 , Ning Chen 4 , Rodney Smith 3 , Chandra Veer Singh 2 , Zhongchao Tan 1, 5 , Yimin A. Wu 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2024-09-02 , DOI: 10.1002/aenm.202402636
Lei Wang 1 , Xue Yao 2 , Holly Fruehwald 3 , Dmitry Akhmetzyanov 3 , Mathew Hanson 1 , Ning Chen 4 , Rodney Smith 3 , Chandra Veer Singh 2 , Zhongchao Tan 1, 5 , Yimin A. Wu 1
Affiliation
|
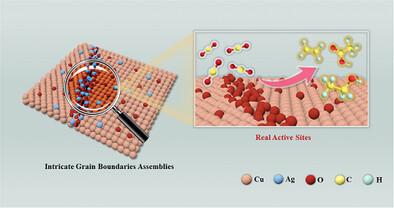
Although intricate structural assemblies contribute to enhancing the activity of electrocatalytic CO2 reduction (ECR) to C2+ products, blindly coupling multiple design strategies may not yield the expected results, and even inhibit the activity of intrinsic catalytic sites. Therefore, elucidating the promoting or inhibitory effects of each design strategy on the CO2-to-C2+ conversion to clarify the real active sites and dynamic oxidation processes is of paramount importance. Here, commonly used grain boundaries (GBs), oxidation states, and alloying strategies are focused on, constructing four different types of catalysts structures: original Cu GBs, oxygen-enriched grain boundary oxidation (GBO), Ag-enriched GBO, and Cu/Ag GBs. Multiple operando characterizations reveal that GBs and GBO strengthen the resistance of the oxidative Cu species to the electrochemical reduction. The in situ generated strongly oxidative hydroxyl radicals alter the local reaction environment on the catalyst surface, inducing and stabilizing oxidative Cuδ+ species. Catalytic activity comparisons indicate that the oxidation state of Cu plays a decisive role in the CO2-to-C2+ conversion, and the nanoalloy effect tends to favor the CH4 production in intricate GBs assemblies. Theoretical calculations suggest that weak CO adsorption on GBO structures facilitates hydrogenation, promoting C–C coupling toward C2+ products.
中文翻译:

揭示 CO2 电还原为 C2+ 产物的复杂晶界组装中的真实活性位点
尽管复杂的结构组件有助于提高电催化 CO2 还原 (ECR) 对 C2+ 产物的活性,但盲目耦合多种设计策略可能无法产生预期结果,甚至会抑制本征催化位点的活性。因此,阐明每种设计策略对 CO2-to-C 2+ 转化的促进或抑制作用以阐明真正的活性位点和动态氧化过程至关重要。本文重点介绍了常用的晶界 (GB)、氧化态和合金化策略,构建了四种不同类型的催化剂结构:原始 Cu GB、富氧晶界氧化 (GBO)、富银 GBO 和 Cu/Ag GB。多个原位表征表明,GB 和 GBO 增强了氧化 Cu 种类对电化学还原的抵抗力。原位生成的强氧化羟基自由基改变了催化剂表面的局部反应环境,诱导和稳定氧化 Cuδ+ 物种。催化活性比较表明,Cu 的氧化态在 CO2 到 C2+ 的转化中起决定性作用,纳米合金效应往往有利于在复杂的 GB s 组件中产生 CH4。理论计算表明,GBO 结构上的 CO 吸附弱促进了氢化,促进了 C-C 偶联到 C2+ 产物。
更新日期:2024-09-02
中文翻译:

揭示 CO2 电还原为 C2+ 产物的复杂晶界组装中的真实活性位点
尽管复杂的结构组件有助于提高电催化 CO2 还原 (ECR) 对 C2+ 产物的活性,但盲目耦合多种设计策略可能无法产生预期结果,甚至会抑制本征催化位点的活性。因此,阐明每种设计策略对 CO2-to-C 2+ 转化的促进或抑制作用以阐明真正的活性位点和动态氧化过程至关重要。本文重点介绍了常用的晶界 (GB)、氧化态和合金化策略,构建了四种不同类型的催化剂结构:原始 Cu GB、富氧晶界氧化 (GBO)、富银 GBO 和 Cu/Ag GB。多个原位表征表明,GB 和 GBO 增强了氧化 Cu 种类对电化学还原的抵抗力。原位生成的强氧化羟基自由基改变了催化剂表面的局部反应环境,诱导和稳定氧化 Cuδ+ 物种。催化活性比较表明,Cu 的氧化态在 CO2 到 C2+ 的转化中起决定性作用,纳米合金效应往往有利于在复杂的 GB s 组件中产生 CH4。理论计算表明,GBO 结构上的 CO 吸附弱促进了氢化,促进了 C-C 偶联到 C2+ 产物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号