当前位置:
X-MOL 学术
›
Eur. J. Heart Fail.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sacubitril/valsartan reduces incident anaemia and iron therapy utilization in heart failure: The PARAGON‐HF trial
European Journal of Heart Failure ( IF 16.9 ) Pub Date : 2024-09-01 , DOI: 10.1002/ejhf.3414 Henri Lu 1, 2 , Brian L Claggett 1 , Milton Packer 3 , Marc A Pfeffer 1 , Carolyn S P Lam 4 , Michael R Zile 5 , Akshay S Desai 1 , Pardeep Jhund 6 , Martin Lefkowitz 7 , John J V McMurray 6 , Scott D Solomon 1 , Muthiah Vaduganathan 1
European Journal of Heart Failure ( IF 16.9 ) Pub Date : 2024-09-01 , DOI: 10.1002/ejhf.3414 Henri Lu 1, 2 , Brian L Claggett 1 , Milton Packer 3 , Marc A Pfeffer 1 , Carolyn S P Lam 4 , Michael R Zile 5 , Akshay S Desai 1 , Pardeep Jhund 6 , Martin Lefkowitz 7 , John J V McMurray 6 , Scott D Solomon 1 , Muthiah Vaduganathan 1
Affiliation
|
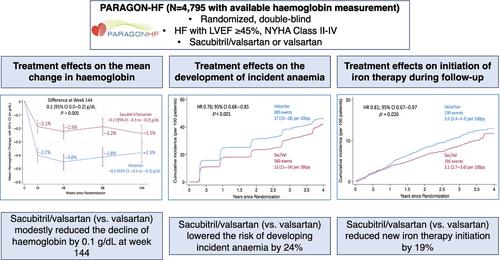
AimsRenin–angiotensin system inhibitors (RASi) have been shown to lower haemoglobin levels, potentially related to reductions in erythropoietin levels and haematopoiesis. We examined whether sacubitril/valsartan might attenuate this effect of RASi alone on incident anaemia in patients with heart failure (HF) with mildly reduced or preserved ejection fraction (HFmrEF/HFpEF).Methods and resultsPARAGON‐HF was a global, multicentre randomized clinical trial of sacubitril/valsartan versus the RASi valsartan in patients with HF and left ventricular ejection fraction ≥45%. We evaluated haemoglobin trajectory and risks of incident anaemia and new iron therapy initiation during follow‐up. Among 4795 participants, 1111 (23.2%) had anaemia at randomization and 5.6% were treated with iron at baseline. Over a median follow‐up of 2.9 years, patients with anaemia were at significantly higher risk for total HF hospitalizations and cardiovascular death, compared with those without anaemia (21.6 vs. 11.5 per 100 patient‐years; adjusted rate ratio 1.31; 95% confidence interval [CI] 1.12–1.54; p = 0.001). Sacubitril/valsartan slightly slowed the decline in haemoglobin levels by 0.1 g/dl (95% CI 0.0–0.2 g/dl; p = 0.005). Participants treated with sacubitril/valsartan were at significantly lower risk of developing anaemia (30.3% vs. 37.6%; hazard ratio [HR] 0.76; 95% CI 0.68–0.85; p < 0.001) and starting iron therapy (8.1% vs. 10.0%; HR 0.81; 95% CI 0.67–0.97; p = 0.026). Treatment effects of sacubitril/valsartan versus valsartan on total HF hospitalizations and cardiovascular death were consistent among patients across the haemoglobin spectrum (p interaction = 0.60).ConclusionsAmong patients with HFmrEF/HFpEF, treatment with sacubitril/valsartan resulted in modestly smaller declines in haemoglobin, lower rates of incident anaemia, and fewer new initiations of iron therapy compared with RASi.Clinical Trial Registration: ClinicalTrials.gov ID NCT01920711.
中文翻译:

沙库巴曲/缬沙坦可减少心力衰竭中贫血的发生和铁剂治疗的使用:PARAGON-HF 试验
目的肾素-血管紧张素系统抑制剂 (RASi) 已被证明可以降低血红蛋白水平,可能与促红细胞生成素水平和造血功能的降低有关。我们研究了沙库巴曲/缬沙坦是否可以减弱单独使用 RASi 对射血分数轻度降低或保留的心力衰竭 (HF) 患者 (HFmrEF/HFpEF) 发生贫血的影响。方法和结果 PARAGON-HF 是一项全球性、多中心随机临床试验沙库巴曲/缬沙坦与 RASi 缬沙坦在心力衰竭且左心室射血分数≥45% 患者中的比较。我们在随访期间评估了血红蛋白轨迹、贫血发生风险以及新铁剂治疗的启动。在 4795 名参与者中,1111 名(23.2%)在随机分组时患有贫血,5.6% 在基线时接受了铁剂治疗。在中位随访 2.9 年中,与无贫血患者相比,贫血患者因心衰住院和心血管死亡的风险明显更高(每 100 患者年 21.6 例 vs. 11.5 例;调整后比率 1.31;95% 置信度)区间 [CI] 1.12–1.54;p = 0.001)。沙库巴曲/缬沙坦使血红蛋白水平下降速度略微减缓 0.1 g/dl(95% CI 0.0–0.2 g/dl;p = 0.005)。接受沙库巴曲/缬沙坦治疗的参与者发生贫血的风险显着降低(30.3% vs. 37.6%;风险比 [HR] 0.76;95% CI 0.68–0.85;p < 0.001)和开始铁剂治疗(8.1% vs. 10.0%;HR 0.81;95% CI 0.67–0.97;p = 0.026)。沙库巴曲/缬沙坦与缬沙坦对心衰住院总次数和心血管死亡的治疗效果在整个血红蛋白谱系的患者中是一致的(pinteraction = 0.60)。结论与 RASi 相比,在 HFmrEF/HFpEF 患者中,沙库巴曲/缬沙坦治疗导致血红蛋白下降幅度较小、贫血发生率较低以及新开始铁剂治疗的次数较少。临床试验注册:ClinicalTrials.gov ID NCT01920711。
更新日期:2024-09-01
中文翻译:

沙库巴曲/缬沙坦可减少心力衰竭中贫血的发生和铁剂治疗的使用:PARAGON-HF 试验
目的肾素-血管紧张素系统抑制剂 (RASi) 已被证明可以降低血红蛋白水平,可能与促红细胞生成素水平和造血功能的降低有关。我们研究了沙库巴曲/缬沙坦是否可以减弱单独使用 RASi 对射血分数轻度降低或保留的心力衰竭 (HF) 患者 (HFmrEF/HFpEF) 发生贫血的影响。方法和结果 PARAGON-HF 是一项全球性、多中心随机临床试验沙库巴曲/缬沙坦与 RASi 缬沙坦在心力衰竭且左心室射血分数≥45% 患者中的比较。我们在随访期间评估了血红蛋白轨迹、贫血发生风险以及新铁剂治疗的启动。在 4795 名参与者中,1111 名(23.2%)在随机分组时患有贫血,5.6% 在基线时接受了铁剂治疗。在中位随访 2.9 年中,与无贫血患者相比,贫血患者因心衰住院和心血管死亡的风险明显更高(每 100 患者年 21.6 例 vs. 11.5 例;调整后比率 1.31;95% 置信度)区间 [CI] 1.12–1.54;p = 0.001)。沙库巴曲/缬沙坦使血红蛋白水平下降速度略微减缓 0.1 g/dl(95% CI 0.0–0.2 g/dl;p = 0.005)。接受沙库巴曲/缬沙坦治疗的参与者发生贫血的风险显着降低(30.3% vs. 37.6%;风险比 [HR] 0.76;95% CI 0.68–0.85;p < 0.001)和开始铁剂治疗(8.1% vs. 10.0%;HR 0.81;95% CI 0.67–0.97;p = 0.026)。沙库巴曲/缬沙坦与缬沙坦对心衰住院总次数和心血管死亡的治疗效果在整个血红蛋白谱系的患者中是一致的(pinteraction = 0.60)。结论与 RASi 相比,在 HFmrEF/HFpEF 患者中,沙库巴曲/缬沙坦治疗导致血红蛋白下降幅度较小、贫血发生率较低以及新开始铁剂治疗的次数较少。临床试验注册:ClinicalTrials.gov ID NCT01920711。































 京公网安备 11010802027423号
京公网安备 11010802027423号