Diabetologia ( IF 8.4 ) Pub Date : 2024-08-31 , DOI: 10.1007/s00125-024-06252-y Fidelma Dunne 1, 2, 3 , Christine Newman 1, 2, 3 , Alberto Alvarez-Iglesias 2 , Paula O'Shea 1 , Declan Devane 1, 2, 4 , Paddy Gillespie 5 , Aoife Egan 1, 6 , Martin O'Donnell 1, 2, 3 , Andrew Smyth 1, 2, 3
|
Aims/hypothesis
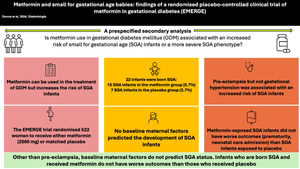
Gestational diabetes mellitus (GDM) is associated with adverse perinatal outcomes because of suboptimal glucose management and glucose control and excessive weight gain. Metformin can offset these factors but is associated with small for gestational age (SGA) infants. We sought to identify risk factors for SGA infants, including the effect of metformin exposure on SGA status.
Methods
In this prespecified secondary analysis of the EMERGE trial, which evaluated the effectiveness of metformin vs placebo in treating GDM and found reduced gestational weight gain and longer time to insulin initiation with metformin use, we included women with a live-born infant and known infant birthweight and gestational age at delivery. We compared the numbers of SGA infants in both groups and explored baseline predictive factors to help identify those at highest risk of delivering an SGA infant.
Results
Baseline maternal characteristics were similar between SGA and non-SGA pregnancies. On multivariable-adjusted regression, no baseline maternal variables were associated with SGA status. Mothers of SGA infants were more likely to develop pre-eclampsia or gestational hypertension (18.2% vs 2.0%, p=0.001; 22.7% vs 5.4%, p=0.005, respectively); after multivariable adjustment, pre-eclampsia was positively associated with SGA status). Among SGA pregnancies, important perinatal outcomes including preterm birth, Caesarean delivery and neonatal care unit admission did not differ between the metformin and placebo groups (20.0% vs 14.3%, p=1.00; 50.0% vs 28.6%, p=0.25; 13.3% vs 42.9%, p=0.27, respectively).
Conclusions/interpretation
Pre-eclampsia was strongly associated with SGA infants. Metformin-exposed SGA infants did not display a more severe SGA phenotype than infants treated with placebo.
Trial registration
Clinical Trials.gov NCT02980276; EudraCT number: 2016-001644-19
Graphical Abstract
中文翻译:

二甲双胍和小于胎龄儿:二甲双胍治疗妊娠糖尿病的随机安慰剂对照临床试验结果 (EMERGE)
目标/假设
妊娠糖尿病 (GDM) 与不良围产期结局相关,因为血糖管理和血糖控制不佳以及体重过度增加。二甲双胍可以抵消这些因素,但与小于胎龄儿 (SGA) 有关。我们试图确定 SGA 婴儿的危险因素,包括二甲双胍暴露对 SGA 状态的影响。
方法
在这项预先指定的 EMERGE 试验的二次分析中,该试验评估了二甲双胍与安慰剂治疗 GDM 的有效性,发现使用二甲双胍可减少妊娠期体重增加和延长胰岛素开始时间,我们纳入了活产婴儿且已知婴儿出生体重和胎龄的妇女分娩。我们比较了两组中 SGA 婴儿的数量,并探讨了基线预测因素,以帮助确定分娩 SGA 婴儿风险最高的婴儿。
结果
SGA 和非 SGA 妊娠的基线母体特征相似。在多变量调整回归中,没有基线母体变量与 SGA 状态相关。SGA 婴儿的母亲更容易患上先兆子痫或妊娠高血压 (分别为 18.2% 和 2.0%,p=0.001;22.7% 和 5.4%,p=0.005);多变量调整后,先兆子痫与 SGA 状态呈正相关)。在 SGA 妊娠中,二甲双胍组和安慰剂组之间的重要围产期结局,包括早产、剖宫产和新生儿监护病房收治率没有差异(分别为 20.0% 对 14.3%,p=1.00;50.0% 对 28.6%,p=0.25;13.3% 对 42.9%,p=0.27)。
结论/解释
子痫前期与 SGA 婴儿密切相关。二甲双胍暴露的 SGA 婴儿没有表现出比安慰剂治疗的婴儿更严重的 SGA 表型。
试用注册
临床 Trials.gov NCT02980276;EudraCT编号:2016-001644-19































 京公网安备 11010802027423号
京公网安备 11010802027423号