当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overcharge protection in aqueous zinc-ion batteries via self-sacrificial additives
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-08-30 , DOI: 10.1039/d4ee01759e Shuo Yang , Liang Mei , Zhuoxi Wu , Jiaxiong Zhu , Pei Li , Hu Hong , Zhiyuan Zeng , Hongfei Li , Funian Mo , Chunyi Zhi
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-08-30 , DOI: 10.1039/d4ee01759e Shuo Yang , Liang Mei , Zhuoxi Wu , Jiaxiong Zhu , Pei Li , Hu Hong , Zhiyuan Zeng , Hongfei Li , Funian Mo , Chunyi Zhi
|
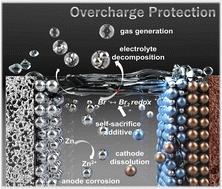
In pursuing zinc-ion batteries (ZIBs) with extended lifetimes, considerable research has been devoted to enhancing their stability, specifically cycling stability, by developing stable cathodes and Zn anodes. However, the durability, that is, reliability of ZIBs under abuse operations, particularly overcharge conditions, has long been overlooked in past research. This work investigates the durability of two typical aqueous ZIBs under overcharge conditions (Mn2+ expanded hydrated vanadium (MnVO) and manganese dioxide (MnO2) as cathode materials). Experimental findings highlight the detrimental effects of overcharging on ZIBs, leading to rapid battery failure primarily attributed to electrolyte decomposition and subsequent deterioration of interfacial contact. Subsequently, self-sacrificial electrolytes are developed by introducing bromine-based additives into the electrolyte (tetrabutylammonium and benzyl trimethylammonium bromine). These additives undergo oxidation before the electrolyte decomposition, introducing an additional Br−/Br2 redox couple. Consequently, this approach effectively stabilizes the electrolyte environment. It provides efficient overcharge protection for extended periods, enabling the Zn‖MnVO and Zn‖MnO2 batteries to sustain for over 650 hours and 550 hours, even under harsh 200% state-of-charge conditions, respectively.
中文翻译:

通过自牺牲添加剂实现水性锌离子电池的过充电保护
为了追求更长寿命的锌离子电池(ZIB),大量研究致力于通过开发稳定的正极和锌负极来提高其稳定性,特别是循环稳定性。然而,耐久性,即ZIB在滥用操作,特别是过度充电条件下的可靠性,在过去的研究中长期以来一直被忽视。这项工作研究了两种典型水性 ZIB 在过充电条件下的耐久性(Mn 2+膨胀水合钒 (MnVO) 和二氧化锰 (MnO 2 ) 作为阴极材料)。实验结果强调了过度充电对 ZIB 的有害影响,导致电池快速失效,主要归因于电解质分解和随后的界面接触恶化。随后,通过将溴基添加剂(四丁基铵和苄基三甲基溴铵)引入电解质中,开发了自牺牲电解质。这些添加剂在电解质分解之前经历氧化,引入额外的 Br - /Br 2氧化还原对。因此,该方法有效地稳定了电解质环境。它提供长时间有效的过充电保护,使 Zn‖MnVO 和 Zn‖MnO 2电池即使在恶劣的 200% 充电状态条件下也能分别维持超过 650 小时和 550 小时。
更新日期:2024-08-30
中文翻译:

通过自牺牲添加剂实现水性锌离子电池的过充电保护
为了追求更长寿命的锌离子电池(ZIB),大量研究致力于通过开发稳定的正极和锌负极来提高其稳定性,特别是循环稳定性。然而,耐久性,即ZIB在滥用操作,特别是过度充电条件下的可靠性,在过去的研究中长期以来一直被忽视。这项工作研究了两种典型水性 ZIB 在过充电条件下的耐久性(Mn 2+膨胀水合钒 (MnVO) 和二氧化锰 (MnO 2 ) 作为阴极材料)。实验结果强调了过度充电对 ZIB 的有害影响,导致电池快速失效,主要归因于电解质分解和随后的界面接触恶化。随后,通过将溴基添加剂(四丁基铵和苄基三甲基溴铵)引入电解质中,开发了自牺牲电解质。这些添加剂在电解质分解之前经历氧化,引入额外的 Br - /Br 2氧化还原对。因此,该方法有效地稳定了电解质环境。它提供长时间有效的过充电保护,使 Zn‖MnVO 和 Zn‖MnO 2电池即使在恶劣的 200% 充电状态条件下也能分别维持超过 650 小时和 550 小时。































 京公网安备 11010802027423号
京公网安备 11010802027423号