当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational dependence of chemical shifts in the proline rich region of TAU protein
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-30 , DOI: 10.1039/d4cp02484b Johannes Stöckelmaier 1 , Chris Oostenbrink 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-30 , DOI: 10.1039/d4cp02484b Johannes Stöckelmaier 1 , Chris Oostenbrink 1
Affiliation
|
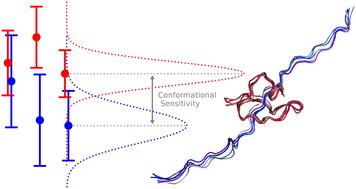
Nuclear magnetic resonance (NMR) is an important method for structure elucidation of proteins, as it is an easily accessible and well understood method. To characterize intrinsically disordered proteins (IDPs) using computational models it is often necessary to analyze and integrate calculated observables with measurements derived from solution NMR experiments. In this case study, we investigate whether and which chemical shifts of the proline-rich region of Tau protein (residues 210–240) offer information about the conformational state to distinguish two different microscopic conformers. Using multiple computational methods, the chemical shifts of these two conformationally distinct structures are calculated. The different methods are compared regarding their ability to compute chemical shifts that are sensitive to conformational change. The analysis of the data shows significant differences between the available methods and gives suggestions for an improved pathway for ensemble reweighting. Nevertheless, the variation in the chemical shifts which are predicted for configurations that are commonly considered to belong to the same conformation is such that this obscures a comparison between distinct conformations. Conformational sensitivity is found for up to ∼26% of calculated chemical shifts. It is found to be unrelated to the atom element and has a minor relationship with the change in the corresponding ϕ dihedral angle.
中文翻译:

TAU 蛋白富含脯氨酸区域化学位移的构象依赖性
核磁共振 (NMR) 是阐明蛋白质结构的重要方法,因为它是一种易于使用且易于理解的方法。为了使用计算模型表征本质无序蛋白质 (IDP),通常需要分析计算的可观测值并将其与溶液 NMR 实验得出的测量值相结合。在本案例研究中,我们研究了 Tau 蛋白富含脯氨酸区域(残基 210-240)是否以及哪些化学位移提供了有关构象状态的信息,以区分两种不同的微观构象异构体。使用多种计算方法,计算这两种构象不同的结构的化学位移。比较了不同方法计算对构象变化敏感的化学位移的能力。数据分析显示了可用方法之间的显着差异,并为集成重新加权的改进途径提供了建议。然而,对于通常被认为属于相同构象的构型预测的化学位移的变化使得这模糊了不同构象之间的比较。高达~26% 的计算化学位移具有构象敏感性。发现它与原子元素无关,与相应的二面角的变化关系较小。
更新日期:2024-09-04
中文翻译:

TAU 蛋白富含脯氨酸区域化学位移的构象依赖性
核磁共振 (NMR) 是阐明蛋白质结构的重要方法,因为它是一种易于使用且易于理解的方法。为了使用计算模型表征本质无序蛋白质 (IDP),通常需要分析计算的可观测值并将其与溶液 NMR 实验得出的测量值相结合。在本案例研究中,我们研究了 Tau 蛋白富含脯氨酸区域(残基 210-240)是否以及哪些化学位移提供了有关构象状态的信息,以区分两种不同的微观构象异构体。使用多种计算方法,计算这两种构象不同的结构的化学位移。比较了不同方法计算对构象变化敏感的化学位移的能力。数据分析显示了可用方法之间的显着差异,并为集成重新加权的改进途径提供了建议。然而,对于通常被认为属于相同构象的构型预测的化学位移的变化使得这模糊了不同构象之间的比较。高达~26% 的计算化学位移具有构象敏感性。发现它与原子元素无关,与相应的二面角的变化关系较小。































 京公网安备 11010802027423号
京公网安备 11010802027423号