当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rethinking Oxygen Redox: Does Oxygen Dimerization Occur without Oxidation in Li2NiO3?
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-08-28 , DOI: 10.1021/acsenergylett.4c02031 Matthew J. W. Ogley 1, 2 , Ashok S. Menon 1, 2 , Beth J. Johnston 1, 2 , Gaurav Pandey 1 , Innes McClelland 2, 3 , Xiaoqun Shi 2, 3 , Stefano Agrestini 4 , Veronica Celorrio 4 , Gabriel E. Pérez 5 , Samuel G. Booth 2, 3 , Jordi Cabana 6 , Serena A. Cussen 2, 7 , Louis F. J. Piper 1, 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-08-28 , DOI: 10.1021/acsenergylett.4c02031 Matthew J. W. Ogley 1, 2 , Ashok S. Menon 1, 2 , Beth J. Johnston 1, 2 , Gaurav Pandey 1 , Innes McClelland 2, 3 , Xiaoqun Shi 2, 3 , Stefano Agrestini 4 , Veronica Celorrio 4 , Gabriel E. Pérez 5 , Samuel G. Booth 2, 3 , Jordi Cabana 6 , Serena A. Cussen 2, 7 , Louis F. J. Piper 1, 2
Affiliation
|
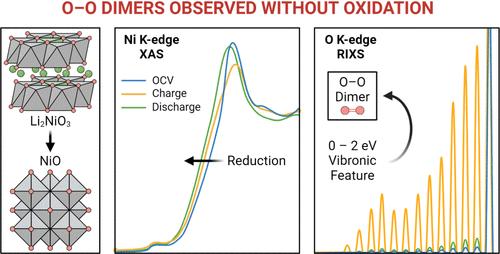
In layered lithium transition metal oxide cathodes, high-voltage operation is accompanied by the formation of oxygen dimers, which are widely used as an indicator of oxygen-redox activity. However, understanding the role that oxygen dimerization plays in facilitating charge compensation is still needed. Li2NiO3 (a 3d8L2-containing compound, where L is a ligand hole) is studied as a model system, where oxygen dimerization is shown to occur without cathode oxidation. Electrochemical cycling results in a net reduction of the cathode, accompanied by structural transformations, despite spectroscopic features of oxygen dimers arising at the top-of-charge. Here, oxygen dimerization is shown to coexist alongside a structurally transformed and electronically reduced cathode structure, thus highlighting that O dimerization is independent of bulk redox processes. This makes it clear that a thermodynamically derived transformation toward a reduced phase remains the only variable capable of generating O–O dimers in Li2NiO3.
中文翻译:

重新思考氧氧化还原:Li2NiO3 中是否会在没有氧化的情况下发生氧二聚?
在层状锂过渡金属氧化物正极中,高电压运行伴随着氧二聚体的形成,氧二聚体被广泛用作氧氧化还原活性的指示剂。然而,仍然需要了解氧二聚在促进电荷补偿中所起的作用。 Li 2 NiO 3 (一种含有 3d 8 L 2的化合物,其中L是配体空穴)作为模型系统进行研究,其中显示氧二聚反应无需阴极氧化即可发生。尽管在充电顶部出现了氧二聚体的光谱特征,但电化学循环导致阴极的净还原,并伴随着结构转变。在这里,氧二聚化与结构转变和电子还原的阴极结构共存,从而强调了O二聚化与本体氧化还原过程无关。这清楚地表明,热力学衍生的向还原相的转变仍然是能够在 Li 2 NiO 3中生成 O-O 二聚体的唯一变量。
更新日期:2024-08-28
中文翻译:

重新思考氧氧化还原:Li2NiO3 中是否会在没有氧化的情况下发生氧二聚?
在层状锂过渡金属氧化物正极中,高电压运行伴随着氧二聚体的形成,氧二聚体被广泛用作氧氧化还原活性的指示剂。然而,仍然需要了解氧二聚在促进电荷补偿中所起的作用。 Li 2 NiO 3 (一种含有 3d 8 L 2的化合物,其中L是配体空穴)作为模型系统进行研究,其中显示氧二聚反应无需阴极氧化即可发生。尽管在充电顶部出现了氧二聚体的光谱特征,但电化学循环导致阴极的净还原,并伴随着结构转变。在这里,氧二聚化与结构转变和电子还原的阴极结构共存,从而强调了O二聚化与本体氧化还原过程无关。这清楚地表明,热力学衍生的向还原相的转变仍然是能够在 Li 2 NiO 3中生成 O-O 二聚体的唯一变量。































 京公网安备 11010802027423号
京公网安备 11010802027423号