当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design of Highly Electrophilic and Stable Metal Nitrido Complexes
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-28 , DOI: 10.1021/acs.accounts.4c00406 Jing Xiang 1 , Huatian Shi 2 , Wai-Lun Man 3 , Tai-Chu Lau 1, 4
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-28 , DOI: 10.1021/acs.accounts.4c00406 Jing Xiang 1 , Huatian Shi 2 , Wai-Lun Man 3 , Tai-Chu Lau 1, 4
Affiliation
|
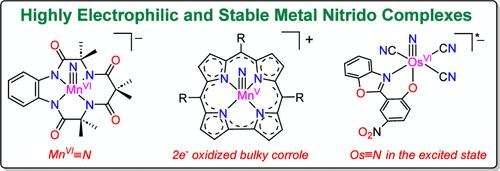
Metal oxo (M═O) and nitrido (M≡N) complexes are two important classes of high-valent transition metal complexes. The use of M═O as oxidants in chemical and biological systems has been extensively investigated. Nature makes use of M═O in enzymes such as cytochrome P450 to oxidize a variety of substrates. Highly oxidizing oxo species have also been synthesized and they have been shown to oxidize organic and inorganic substrates via one-electron oxidation, O atom transfer, and H atom abstraction pathways. In contrast, the oxidation chemistry of M≡N is much less investigated. Although a variety of nitrido complexes are known, most of them are inert and do not show appreciable oxidizing properties, which is not unexpected since the N3– ligand is much more electron-donating than the O2– ligand. In principle, highly electrophilic/oxidizing nitrido complexes may be designed by using weakly coordinating ancillary ligands and/or by increasing the oxidation state of the metal centers. A number of such species have been generated in solution at low temperatures. However, attempts to isolate them are often hampered by their ease of decomposition via bimolecular N···N coupling to generate N2. In some cases, decomposition occurs by intramolecular nitrogenation of the ancillary ligand.
中文翻译:

高亲电且稳定的金属氮化物配合物的设计
金属氧代(M=O)和硝基(M=N)配合物是两类重要的高价过渡金属配合物。 M=O 作为化学和生物系统中的氧化剂的用途已被广泛研究。大自然利用细胞色素P450等酶中的 M=O 来氧化多种底物。还合成了高氧化性的含氧物质,并且它们已被证明可以通过单电子氧化、O原子转移和H原子提取途径氧化有机和无机底物。相比之下,MeqN 的氧化化学研究较少。尽管已知有多种氮化物络合物,但它们中的大多数是惰性的并且不表现出明显的氧化特性,这并不意外,因为N 3-配体比O 2-配体更具电子供给性。原则上,可以通过使用弱配位辅助配体和/或通过增加金属中心的氧化态来设计高亲电/氧化性氮化物络合物。许多这样的物质已在低温溶液中产生。然而,分离它们的尝试常常受到阻碍,因为它们容易通过双分子N·N耦合生成N 2分解。在某些情况下,分解是通过辅助配体的分子内氮化而发生的。
更新日期:2024-08-28
中文翻译:

高亲电且稳定的金属氮化物配合物的设计
金属氧代(M=O)和硝基(M=N)配合物是两类重要的高价过渡金属配合物。 M=O 作为化学和生物系统中的氧化剂的用途已被广泛研究。大自然利用细胞色素P450等酶中的 M=O 来氧化多种底物。还合成了高氧化性的含氧物质,并且它们已被证明可以通过单电子氧化、O原子转移和H原子提取途径氧化有机和无机底物。相比之下,MeqN 的氧化化学研究较少。尽管已知有多种氮化物络合物,但它们中的大多数是惰性的并且不表现出明显的氧化特性,这并不意外,因为N 3-配体比O 2-配体更具电子供给性。原则上,可以通过使用弱配位辅助配体和/或通过增加金属中心的氧化态来设计高亲电/氧化性氮化物络合物。许多这样的物质已在低温溶液中产生。然而,分离它们的尝试常常受到阻碍,因为它们容易通过双分子N·N耦合生成N 2分解。在某些情况下,分解是通过辅助配体的分子内氮化而发生的。































 京公网安备 11010802027423号
京公网安备 11010802027423号