Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4-Methoxypicolinic Acid N-Oxide: One of the Shortest Hydrogen Bonds Known Characterized by Neutron Diffraction, Inelastic Neutron Scattering, Infrared Spectroscopy, and Periodic DFT Calculations
ACS Omega ( IF 3.7 ) Pub Date : 2024-08-29 , DOI: 10.1021/acsomega.4c05344
Jernej Stare 1 , Jože Grdadolnik 1 , Sax Mason 2 , Alberto Albinati 3 , Juergen Eckert 4
ACS Omega ( IF 3.7 ) Pub Date : 2024-08-29 , DOI: 10.1021/acsomega.4c05344
Jernej Stare 1 , Jože Grdadolnik 1 , Sax Mason 2 , Alberto Albinati 3 , Juergen Eckert 4
Affiliation
|
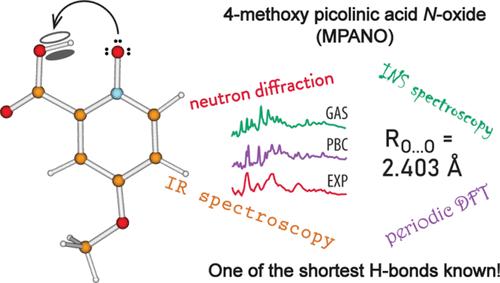
The present work focuses on the case of an extremely short intramolecular O–H···O hydrogen bond (H-bond) found in 4-methoxypicolinic acid N-oxide (MPANO). The donor···acceptor separation of 2.403 Å makes the H-bond in MPANO one of the shortest H-bonds known. We elucidated the structure and dynamics of the H-bond by two neutron-based techniques, namely, single-crystal diffraction and inelastic scattering (INS) vibrational spectroscopy. We also utilized conventional infrared (IR) spectroscopy as well as quantum chemical computations on isolated and periodic models. Both the protiated and deuterated variants of MPANO were investigated by INS and IR. All the methods used unequivocally confirm the existence of an extremely short, asymmetric H-bond, with the proton located near yet off the midpoint. The main relevant feature of the IR spectrum is an extremely broad, complex, and red-shifted OH (OD) stretching band spanning between 1800 and 500 cm–1 and centered at about 1360 cm–1, which indicates the presence of extensive anharmonicity and coupling with other H-bond modes. Of the modes characteristic of H-bond dynamics, only the out-of-plane OH (OD) bending can clearly be detected in the INS spectra; it has a relatively high frequency indicative of the strength of the H-bond. The computed structure is in excellent agreement with the diffraction measurement when periodicity is taken into account. The calculated harmonic frequencies show a reasonable match with the observed spectral features, whereby the assignment of the IR and INS spectra is facilitated. The hydrogen stretching frequency, however, appears to be significantly overestimated, on account of the limitations of the harmonic approximation and the complex nature of the short H-bond.
中文翻译:

4-甲氧基吡啶甲酸 N-氧化物:已知最短的氢键之一,可通过中子衍射、非弹性中子散射、红外光谱和定期 DFT 计算来表征
目前的工作重点是在 4-甲氧基吡啶甲酸N-氧化物 (MPANO) 中发现的极短分子内 O-H·O 氢键 (H-bond) 的情况。 2.403 Å 的供体·受体间距使得 MPANO 中的氢键成为已知的最短氢键之一。我们通过两种基于中子的技术,即单晶衍射和非弹性散射(INS)振动光谱,阐明了氢键的结构和动力学。我们还利用了传统的红外 (IR) 光谱以及对孤立和周期性模型的量子化学计算。通过 INS 和 IR 研究了 MPANO 的质子化和氘化变体。使用的所有方法都明确证实了极短的不对称氢键的存在,其中质子位于中点附近但偏离。红外光谱的主要相关特征是极其宽广、复杂且红移的 OH (OD) 伸缩带,跨越 1800 至 500 cm –1 ,中心位于约 1360 cm –1 ,这表明存在广泛的非谐性和与其他氢键模式耦合。在氢键动力学的模式特征中,只有面外 OH (OD) 弯曲可以在 INS 光谱中清晰地检测到;它具有相对较高的频率来指示氢键的强度。当考虑周期性时,计算的结构与衍射测量非常一致。计算出的谐波频率与观察到的光谱特征合理匹配,从而有利于红外和惯性导航光谱的分配。 然而,由于调和近似的限制和短氢键的复杂性质,氢伸缩频率似乎被严重高估。
更新日期:2024-08-29
中文翻译:

4-甲氧基吡啶甲酸 N-氧化物:已知最短的氢键之一,可通过中子衍射、非弹性中子散射、红外光谱和定期 DFT 计算来表征
目前的工作重点是在 4-甲氧基吡啶甲酸N-氧化物 (MPANO) 中发现的极短分子内 O-H·O 氢键 (H-bond) 的情况。 2.403 Å 的供体·受体间距使得 MPANO 中的氢键成为已知的最短氢键之一。我们通过两种基于中子的技术,即单晶衍射和非弹性散射(INS)振动光谱,阐明了氢键的结构和动力学。我们还利用了传统的红外 (IR) 光谱以及对孤立和周期性模型的量子化学计算。通过 INS 和 IR 研究了 MPANO 的质子化和氘化变体。使用的所有方法都明确证实了极短的不对称氢键的存在,其中质子位于中点附近但偏离。红外光谱的主要相关特征是极其宽广、复杂且红移的 OH (OD) 伸缩带,跨越 1800 至 500 cm –1 ,中心位于约 1360 cm –1 ,这表明存在广泛的非谐性和与其他氢键模式耦合。在氢键动力学的模式特征中,只有面外 OH (OD) 弯曲可以在 INS 光谱中清晰地检测到;它具有相对较高的频率来指示氢键的强度。当考虑周期性时,计算的结构与衍射测量非常一致。计算出的谐波频率与观察到的光谱特征合理匹配,从而有利于红外和惯性导航光谱的分配。 然而,由于调和近似的限制和短氢键的复杂性质,氢伸缩频率似乎被严重高估。


































 京公网安备 11010802027423号
京公网安备 11010802027423号