当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Polyethylene oxide-based solid-state polymer electrolyte hybridized with liquid catholyte for semi-solid-state rechargeable Mg–O2 batteries
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-08-28 , DOI: 10.1039/d4ta04244a Ayan Sarkar , Shang-Yang Huang , Vasantan Rasupillai Dharmaraj , Behrouz Bazri , Kevin Iputera , Hsiu-Hui Su , Yi-An Chen , Han-Chen Chen , Yu-Ping Lin , Ren-Jei Chung , Da-Hua Wei , Ru-Shi Liu
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-08-28 , DOI: 10.1039/d4ta04244a Ayan Sarkar , Shang-Yang Huang , Vasantan Rasupillai Dharmaraj , Behrouz Bazri , Kevin Iputera , Hsiu-Hui Su , Yi-An Chen , Han-Chen Chen , Yu-Ping Lin , Ren-Jei Chung , Da-Hua Wei , Ru-Shi Liu
|
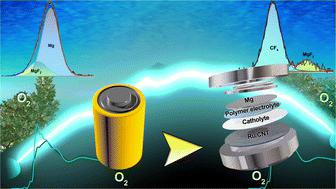
In this article, we report the fabrication of a free-standing non-porous polyethylene oxide (PEO)-based solid-state polymer electrolyte with the amalgamation of magnesium triflate (Mg(OTf)2) and plasticizer succinonitrile (SN) for room-temperature secondary Mg–O2 batteries. The polymer electrolyte comprising [EO] : Mg2+ = 20 : 1 and 30 wt% SN delivered the highest room-temperature (RT) conductivity of 3.9 × 10−5 S cm−1, which reached 2.2 × 10−4 S cm−1 at 60 °C. Ruthenium nanoparticles anchored multi-walled carbon nanotubes (Ru/CNT) on carbon paper were used as cathode catalysts for rechargeable Mg–O2 batteries. The non-establishment of the cathode–solid-state polymer electrolyte interphase was alleviated by introducing a separator soaked with 50–60 μL of 1 M Mg(TFSI)2 in diglyme (G2) between the polymer electrolyte and Ru/CNT cathode catalyst. The Mg–O2 battery with this hybrid electrolyte configuration delivered a deep discharge capacity of 9489 mA h g−1 at 100 mA g−1 and a stable galvanostatic discharge–charge performance with the cycle number reaching 51 at 100 mA g−1 with 500 mA h g−1 curtailing capacity, demonstrating an average charging–discharging voltage hysteresis of 1.14 V at RT. The polymer electrolyte also participated in forming an MgF2-rich stable solid-electrolyte interphase layer on the Mg anode, substantially protecting the anode surface from severe side reactions and corrosion. Raman spectroscopy and distribution of relaxation time studies shed light on the probable mechanism of the liquid catholyte in improving the interphase issue.
中文翻译:

用于半固态可充电Mg-O2电池的聚环氧乙烷基固态聚合物电解质与液体阴极电解液混合
在本文中,我们报道了一种独立式无孔聚环氧乙烷(PEO)基固态聚合物电解质的制造,其中混合了三氟甲磺酸镁(Mg(OTf) 2 )和增塑剂丁二腈(SN),用于室温温度二次Mg-O 2电池。含有[EO] : Mg 2+ = 20 : 1和30 wt% SN的聚合物电解质的室温电导率最高为3.9 × 10 -5 S cm -1 ,达到2.2 × 10 -4 S cm 60°C 时为-1 。将多壁碳纳米管(Ru/CNT)锚定在碳纸上的钌纳米颗粒用作可充电Mg-O 2电池的阴极催化剂。通过在聚合物电解质和 Ru/CNT 阴极催化剂之间引入浸泡有 50-60 μL 1 M Mg(TFSI) 2的二甘醇二甲醚 (G2) 的隔膜,可以缓解阴极 - 固态聚合物电解质界面不形成的问题。具有这种混合电解质配置的Mg-O 2电池在100 mA g -1下具有9489 mA hg -1的深度放电容量和稳定的恒电流放电-充电性能,循环次数在100 mA g -1下达到51次,循环次数为500次。 mA hg -1减少了容量,表明室温下的平均充放电电压滞后为 1.14 V。 聚合物电解质还参与在镁阳极上形成富含MgF 2的稳定固体电解质界面层,基本上保护阳极表面免受严重的副反应和腐蚀。拉曼光谱和弛豫时间分布研究揭示了液体阴极电解液改善相间问题的可能机制。
更新日期:2024-08-28
中文翻译:

用于半固态可充电Mg-O2电池的聚环氧乙烷基固态聚合物电解质与液体阴极电解液混合
在本文中,我们报道了一种独立式无孔聚环氧乙烷(PEO)基固态聚合物电解质的制造,其中混合了三氟甲磺酸镁(Mg(OTf) 2 )和增塑剂丁二腈(SN),用于室温温度二次Mg-O 2电池。含有[EO] : Mg 2+ = 20 : 1和30 wt% SN的聚合物电解质的室温电导率最高为3.9 × 10 -5 S cm -1 ,达到2.2 × 10 -4 S cm 60°C 时为-1 。将多壁碳纳米管(Ru/CNT)锚定在碳纸上的钌纳米颗粒用作可充电Mg-O 2电池的阴极催化剂。通过在聚合物电解质和 Ru/CNT 阴极催化剂之间引入浸泡有 50-60 μL 1 M Mg(TFSI) 2的二甘醇二甲醚 (G2) 的隔膜,可以缓解阴极 - 固态聚合物电解质界面不形成的问题。具有这种混合电解质配置的Mg-O 2电池在100 mA g -1下具有9489 mA hg -1的深度放电容量和稳定的恒电流放电-充电性能,循环次数在100 mA g -1下达到51次,循环次数为500次。 mA hg -1减少了容量,表明室温下的平均充放电电压滞后为 1.14 V。 聚合物电解质还参与在镁阳极上形成富含MgF 2的稳定固体电解质界面层,基本上保护阳极表面免受严重的副反应和腐蚀。拉曼光谱和弛豫时间分布研究揭示了液体阴极电解液改善相间问题的可能机制。





































 京公网安备 11010802027423号
京公网安备 11010802027423号