当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Study of degradation mechanisms in aqueous-processed Ni-rich cathodes for enhanced sustainability of batteries
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-08-27 , DOI: 10.1039/d4ta03592e Heyin Chen 1 , Agnes-Matilda Mattsson 1 , Laura King 1 , Haidong Liu 1 , Ida Nielsen 1 , Tove Ericson 1 , Alexei Preobrajenski 2 , William R. Brant 1 , Maria Hahlin 1, 3
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-08-27 , DOI: 10.1039/d4ta03592e Heyin Chen 1 , Agnes-Matilda Mattsson 1 , Laura King 1 , Haidong Liu 1 , Ida Nielsen 1 , Tove Ericson 1 , Alexei Preobrajenski 2 , William R. Brant 1 , Maria Hahlin 1, 3
Affiliation
|
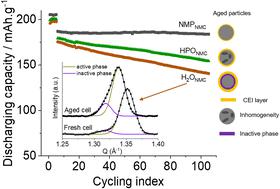
Traditionally, Ni-rich-layered oxide cathodes for lithium-ion batteries are produced utilizing N-methyl-2-pyrrolidone (NMP)-processed casting. However, to avoid using the reprotoxic solvent NMP, aqueous processing becomes one of the options. In this study, H2O-processed LiNi0.8Mn0.1Co0.1O2 (NMC811) electrodes have been prepared to compare with the NMP-processed counterparts to investigate the degradation mechanism. The thick cathode–electrolyte interphase (CEI), NiO-like phase formation, and the growth of electrochemically inactive NMC particles after long-term cycling lead to capacity decay. In addition, phosphoric acid (H3PO4) was utilized to lower the pH value during the water-processed electrode preparation, to avoid corrosion of the aluminium current collector. The use of H3PO4 enhanced the capacity retention of NMC811 electrodes, likely owing to the formation of a LiF-rich CEI layer in the initial cycle(s) and the alleviated formation of electrochemically inactive NMC particles. Additionally, reaction inhomogeneity is present in H3PO4-modified electrodes, which is attributed to various Li-ion reinsertion resistances throughout the porous electrode during long-term cycling. Although the performance of the water-processed NMC811 electrode is not reaching the level of NMP-processed electrodes, this study provides key insights into the involved degradation mechanisms and demonstrates a viable pathway for the development of sustainable battery manufacturing processes.
中文翻译:

研究水处理富镍正极的降解机制以增强电池的可持续性
传统上,锂离子电池的富镍层状氧化物阴极是利用N-甲基-2-吡咯烷酮 (NMP) 加工的铸造工艺生产的。然而,为了避免使用生殖毒性溶剂 NMP,水处理成为选择之一。在本研究中,制备了H 2 O处理的LiNi 0.8 Mn 0.1 Co 0.1 O 2 (NMC811)电极,与NMP处理的电极进行比较,以研究其降解机制。长期循环后,厚的阴极-电解质界面(CEI)、类NiO相的形成以及电化学不活泼的NMC颗粒的生长导致容量衰减。此外,在水处理电极制备过程中,利用磷酸(H 3 PO 4 )降低pH值,以避免铝集流体的腐蚀。 H 3 PO 4的使用增强了NMC811电极的容量保持率,这可能是由于在初始循环中形成了富含LiF的CEI层并且减少了电化学惰性NMC颗粒的形成。此外,H 3 PO 4改性电极中存在反应不均匀性,这是由于长期循环期间整个多孔电极中的各种锂离子再插入电阻造成的。 尽管水处理的NMC811电极的性能未达到NMP处理的电极的水平,但这项研究提供了对所涉及的降解机制的重要见解,并展示了开发可持续电池制造工艺的可行途径。
更新日期:2024-08-27
中文翻译:

研究水处理富镍正极的降解机制以增强电池的可持续性
传统上,锂离子电池的富镍层状氧化物阴极是利用N-甲基-2-吡咯烷酮 (NMP) 加工的铸造工艺生产的。然而,为了避免使用生殖毒性溶剂 NMP,水处理成为选择之一。在本研究中,制备了H 2 O处理的LiNi 0.8 Mn 0.1 Co 0.1 O 2 (NMC811)电极,与NMP处理的电极进行比较,以研究其降解机制。长期循环后,厚的阴极-电解质界面(CEI)、类NiO相的形成以及电化学不活泼的NMC颗粒的生长导致容量衰减。此外,在水处理电极制备过程中,利用磷酸(H 3 PO 4 )降低pH值,以避免铝集流体的腐蚀。 H 3 PO 4的使用增强了NMC811电极的容量保持率,这可能是由于在初始循环中形成了富含LiF的CEI层并且减少了电化学惰性NMC颗粒的形成。此外,H 3 PO 4改性电极中存在反应不均匀性,这是由于长期循环期间整个多孔电极中的各种锂离子再插入电阻造成的。 尽管水处理的NMC811电极的性能未达到NMP处理的电极的水平,但这项研究提供了对所涉及的降解机制的重要见解,并展示了开发可持续电池制造工艺的可行途径。





































 京公网安备 11010802027423号
京公网安备 11010802027423号