当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating gas–surface interactions relevant to atmospheric particle growth using combined temperature programmed desorption and temperature-dependent uptake
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-28 , DOI: 10.1039/d4cp02528h Kristen N Johnson 1 , Yixin Li 1 , Michael J Ezell 1 , Pascale S J Lakey 1 , Manabu Shiraiwa 1 , Barbara J Finlayson-Pitts 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-28 , DOI: 10.1039/d4cp02528h Kristen N Johnson 1 , Yixin Li 1 , Michael J Ezell 1 , Pascale S J Lakey 1 , Manabu Shiraiwa 1 , Barbara J Finlayson-Pitts 1
Affiliation
|
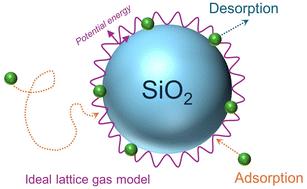
Understanding growth mechanisms for particles in air is fundamental to developing a predictive capability for their impacts on human health, visibility, and climate. In the case of highly viscous semi-solid or solid particles, the likelihood of impinging gases being taken up to grow the particle will be influenced by the initial uptake coefficient and by the residence time of the adsorbed gas on the surface. Here, a new approach that combines Knudsen cell capabilities for gas uptake measurements with temperature programmed desorption (TPD) for binding energy measurements of gases is described. The application of this unique capability to the uptake of organic gases on silica demonstrates its utility and the combination of thermodynamic and kinetic data that can be obtained. Lower limits to the initial net uptake coefficients at 170 K are (3.0 ± 0.6) × 10−3, (4.9 ± 0.6) × 10−3 and (4.3 ± 0.8) × 10−3 for benzene, 1-chloropentane, and methanol, respectively, and are reported here for the first time. The uptake data demonstrated that the ideal gas lattice model was appropriate, which informed the analysis of the TPD data. From the thermal desorption measurements, desorption energies of 34.6 ± 2.5, 45.8 ± 5.5, and 40.0 ± 5.6 kJ mol−1 (errors are 1σ) are obtained for benzene, 1-chloropentane, and methanol, respectively, and show good agreement with previously reported measurements. A multiphase kinetics model was applied to quantify uptake, desorption, and diffusion through the particle multilayers and hence extract desorption kinetics. Implications for uptake of organics on silica surfaces in the atmosphere and the utility of this system for determining relationships between residence times of organic gases and particle surfaces of varying composition are discussed in the context of developing quantitative predictions for growth of aerosol particles in air.
中文翻译:

使用组合程序升温解吸和温度依赖性吸收来阐明与大气颗粒生长相关的气体-表面相互作用
了解空气中颗粒的生长机制对于开发其对人类健康、能见度和气候影响的预测能力至关重要。在高粘性半固体或固体颗粒的情况下,吸收冲击气体以生长颗粒的可能性将受到初始吸收系数和吸附气体在表面上的停留时间的影响。本文描述了一种新方法,该方法将用于气体吸收测量的努森池功能与用于气体结合能测量的程序升温解吸 (TPD) 功能相结合。这种独特的能力在二氧化硅上吸收有机气体的应用证明了它的实用性以及可以获得的热力学和动力学数据的结合。对于苯、1-氯戊烷和甲醇,170 K 时初始净吸收系数的下限为 (3.0 ± 0.6) × 10 -3 、(4.9 ± 0.6) × 10 -3和 (4.3 ± 0.8) × 10 -3分别是,并且是首次在这里报道。吸收数据表明理想气体晶格模型是合适的,这为 TPD 数据的分析提供了信息。通过热解吸测量,得到苯、1-氯戊烷和甲醇的解吸能分别为 34.6 ± 2.5、45.8 ± 5.5 和 40.0 ± 5.6 kJ mol −1 (误差为 1σ),与之前的结果吻合良好报告的测量值。应用多相动力学模型来量化颗粒多层的吸收、解吸和扩散,从而提取解吸动力学。 在开发空气中气溶胶颗粒生长的定量预测的背景下,讨论了大气中二氧化硅表面吸收有机物的影响,以及该系统用于确定有机气体停留时间与不同组成的颗粒表面之间关系的实用性。
更新日期:2024-08-28
中文翻译:

使用组合程序升温解吸和温度依赖性吸收来阐明与大气颗粒生长相关的气体-表面相互作用
了解空气中颗粒的生长机制对于开发其对人类健康、能见度和气候影响的预测能力至关重要。在高粘性半固体或固体颗粒的情况下,吸收冲击气体以生长颗粒的可能性将受到初始吸收系数和吸附气体在表面上的停留时间的影响。本文描述了一种新方法,该方法将用于气体吸收测量的努森池功能与用于气体结合能测量的程序升温解吸 (TPD) 功能相结合。这种独特的能力在二氧化硅上吸收有机气体的应用证明了它的实用性以及可以获得的热力学和动力学数据的结合。对于苯、1-氯戊烷和甲醇,170 K 时初始净吸收系数的下限为 (3.0 ± 0.6) × 10 -3 、(4.9 ± 0.6) × 10 -3和 (4.3 ± 0.8) × 10 -3分别是,并且是首次在这里报道。吸收数据表明理想气体晶格模型是合适的,这为 TPD 数据的分析提供了信息。通过热解吸测量,得到苯、1-氯戊烷和甲醇的解吸能分别为 34.6 ± 2.5、45.8 ± 5.5 和 40.0 ± 5.6 kJ mol −1 (误差为 1σ),与之前的结果吻合良好报告的测量值。应用多相动力学模型来量化颗粒多层的吸收、解吸和扩散,从而提取解吸动力学。 在开发空气中气溶胶颗粒生长的定量预测的背景下,讨论了大气中二氧化硅表面吸收有机物的影响,以及该系统用于确定有机气体停留时间与不同组成的颗粒表面之间关系的实用性。































 京公网安备 11010802027423号
京公网安备 11010802027423号