当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dirhodium(II) complex catalyzed dehydrosilylation of styrenes: theoretical investigations on the mechanism, selectivity, and ligand effects
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-28 , DOI: 10.1039/d4cp02576h Ziying Zhong 1 , Qingzhong Li 2 , Xiaoyan Li 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-28 , DOI: 10.1039/d4cp02576h Ziying Zhong 1 , Qingzhong Li 2 , Xiaoyan Li 1
Affiliation
|
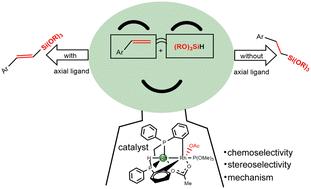
The dirhodium(II) complexes with bridging phosphine and OAc ligands showed high reactivity and selectivities in olefin dehydrosilylation. In order to determine the structure of the actual catalyst which cannot be determined experimentally, the geometries of the dirhodium catalyst, the detailed catalytic mechanism, and the stereo- and chemo-selectivities of the title reaction were studied using DFT calculations. The results showed that one OAc group is monodentate and the other is bidentate in the dirhodium catalyst C′. The determined catalytic cycle consists of four processes: Rh–H bond activation in C′, Si–H bond activation in alkoxysilane, alkylene insertion into the Rh–Si bond, followed by β-H elimination or σ-metathesis reaction. Among them, the alkylene insertion process is the rate-determining step. The stereoselectivity of the title reaction is controlled by the steric effect and orbital interactions between the alkyene and dirhodium catalysts in the β-H elimination process. The chemoselectivity is regulated by the presence of the axial ligand in the dirhodium catalyst, when there is an axial ligand coordinated to the Rh atom, E-alkene is the main product, whereas alkane would be obtained in the absence of an axial ligand. Our work determines the structure of the actual catalyst, and provides explanations and predictions for the activity, and chemo- and stereo-selectivity control of olefin dehydrosilylation.
中文翻译:

二铑(II)配合物催化苯乙烯脱氢硅烷化:机理、选择性和配体效应的理论研究
具有桥联膦和OAc配体的二铑( II )络合物在烯烃脱氢硅烷化中表现出高反应活性和选择性。为了确定无法通过实验确定的实际催化剂的结构,使用 DFT 计算研究了二铑催化剂的几何形状、详细的催化机理以及标题反应的立体选择性和化学选择性。结果表明二铑催化剂C'中一个OAc基团是单齿基团,另一个是二齿基团。确定的催化循环由四个过程组成: C'中的Rh-H键活化、烷氧基硅烷中的Si-H键活化、亚烷基插入到Rh-Si键中,然后进行β-H消除或σ-复分解反应。其中,亚烷基插入过程是速率决定步骤。标题反应的立体选择性由β-H消除过程中炔烃和二铑催化剂之间的空间效应和轨道相互作用控制。化学选择性是通过二铑催化剂中轴向配体的存在来调节的,当存在与Rh原子配位的轴向配体时, E-烯烃是主要产物,而在没有轴向配体的情况下将得到烷烃。我们的工作确定了实际催化剂的结构,并为烯烃脱氢硅烷化的活性以及化学和立体选择性控制提供了解释和预测。
更新日期:2024-08-28
中文翻译:

二铑(II)配合物催化苯乙烯脱氢硅烷化:机理、选择性和配体效应的理论研究
具有桥联膦和OAc配体的二铑( II )络合物在烯烃脱氢硅烷化中表现出高反应活性和选择性。为了确定无法通过实验确定的实际催化剂的结构,使用 DFT 计算研究了二铑催化剂的几何形状、详细的催化机理以及标题反应的立体选择性和化学选择性。结果表明二铑催化剂C'中一个OAc基团是单齿基团,另一个是二齿基团。确定的催化循环由四个过程组成: C'中的Rh-H键活化、烷氧基硅烷中的Si-H键活化、亚烷基插入到Rh-Si键中,然后进行β-H消除或σ-复分解反应。其中,亚烷基插入过程是速率决定步骤。标题反应的立体选择性由β-H消除过程中炔烃和二铑催化剂之间的空间效应和轨道相互作用控制。化学选择性是通过二铑催化剂中轴向配体的存在来调节的,当存在与Rh原子配位的轴向配体时, E-烯烃是主要产物,而在没有轴向配体的情况下将得到烷烃。我们的工作确定了实际催化剂的结构,并为烯烃脱氢硅烷化的活性以及化学和立体选择性控制提供了解释和预测。

































 京公网安备 11010802027423号
京公网安备 11010802027423号