当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proton-coupled electron transfer at a mis-metalated zinc site detected with protein charge ladders
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-28 , DOI: 10.1039/d4cp01989j Mayte Gonzalez 1 , Matthew J Guberman-Pfeffer 1 , Jordan C Koone 1 , Chad M Dashnaw 1 , Travis J Lato 1 , Bryan F Shaw 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-28 , DOI: 10.1039/d4cp01989j Mayte Gonzalez 1 , Matthew J Guberman-Pfeffer 1 , Jordan C Koone 1 , Chad M Dashnaw 1 , Travis J Lato 1 , Bryan F Shaw 1
Affiliation
|
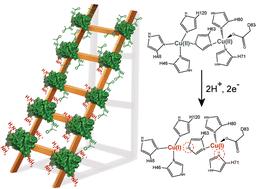
Distinguishing proton-coupled electron transfer (PCET) from uncoupled electron transfer (ET) in proteins can be challenging. A recent investigation [J. C. Koone, M. Simmang, D. L. Saenger, M. L. Hunsicker-Wang and B. F. Shaw, J. Am. Chem. Soc., 145, 16488–16497] reported that protein charge ladders and capillary electrophoresis can distinguish between single ET, PCET, and two-proton coupled ET (2PCET) by directly measuring the change in protein net charge upon reduction/oxidation (ΔZET). The current study used similar methods to assess PCET in zinc-free, “double copper” superoxide dismutase-1 (4Cu-SOD1), where one copper is bound at the copper site of each monomer and one copper is bound at the bridging zinc site, resulting in a quasi-type III Cu center. At pH 7.4, the net charge (Z) of the 4Cu-SOD1 dimer was unaffected by reduction of all four Cu2+ ions, i.e., ΔZ4ET = −0.09 ± 0.05 per dimer (−0.02 ± 0.01 per copper atom). These values suggest that PCET is taking place at all four Cu atoms of the homodimer. Molecular dynamics and Poisson–Boltzmann calculations suggest that a metal-coordinating histidine at the zinc site (His71) is the proton acceptor. These data show how ligands of a naturally occurring zinc site can help facilitate PCET when the right redox metal is bound.
中文翻译:

用蛋白质电荷梯检测到错金属化锌位点的质子耦合电子转移
区分蛋白质中的质子耦合电子转移 (PCET) 和非耦合电子转移 (ET) 可能具有挑战性。最近的一项调查 [JC Koone、M. Simmang、DL Saenger、ML Hunsicker-Wang 和 BF Shaw、 J. Am。化学。苏克。 , 145 , 16488–16497]报道蛋白质电荷梯和毛细管电泳可以通过直接测量还原/氧化时蛋白质净电荷的变化(Δ Z ET )来区分单 ET、PCET 和双质子耦合 ET (2PCET) 。目前的研究使用类似的方法来评估无锌“双铜”超氧化物歧化酶-1 (4Cu-SOD1) 中的 PCET,其中一个铜结合在每个单体的铜位点,一个铜结合在桥接锌位点,产生准 III 型 Cu 中心。在 pH 7.4 时,4Cu-SOD1 二聚体的净电荷 ( Z ) 不受所有四个 Cu 2+离子还原的影响,即Δ Z 4ET = 每个二聚体 -0.09 ± 0.05(每个铜原子 -0.02 ± 0.01)。这些值表明 PCET 发生在同二聚体的所有四个 Cu 原子上。分子动力学和泊松-玻尔兹曼计算表明,锌位点上的金属配位组氨酸 (His71) 是质子受体。这些数据表明,当结合正确的氧化还原金属时,天然锌位点的配体如何帮助促进 PCET。
更新日期:2024-08-28
中文翻译:

用蛋白质电荷梯检测到错金属化锌位点的质子耦合电子转移
区分蛋白质中的质子耦合电子转移 (PCET) 和非耦合电子转移 (ET) 可能具有挑战性。最近的一项调查 [JC Koone、M. Simmang、DL Saenger、ML Hunsicker-Wang 和 BF Shaw、 J. Am。化学。苏克。 , 145 , 16488–16497]报道蛋白质电荷梯和毛细管电泳可以通过直接测量还原/氧化时蛋白质净电荷的变化(Δ Z ET )来区分单 ET、PCET 和双质子耦合 ET (2PCET) 。目前的研究使用类似的方法来评估无锌“双铜”超氧化物歧化酶-1 (4Cu-SOD1) 中的 PCET,其中一个铜结合在每个单体的铜位点,一个铜结合在桥接锌位点,产生准 III 型 Cu 中心。在 pH 7.4 时,4Cu-SOD1 二聚体的净电荷 ( Z ) 不受所有四个 Cu 2+离子还原的影响,即Δ Z 4ET = 每个二聚体 -0.09 ± 0.05(每个铜原子 -0.02 ± 0.01)。这些值表明 PCET 发生在同二聚体的所有四个 Cu 原子上。分子动力学和泊松-玻尔兹曼计算表明,锌位点上的金属配位组氨酸 (His71) 是质子受体。这些数据表明,当结合正确的氧化还原金属时,天然锌位点的配体如何帮助促进 PCET。































 京公网安备 11010802027423号
京公网安备 11010802027423号