当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simulation of the non-adiabatic dynamics of an enone-Lewis acid complex in an explicit solvent
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-28 , DOI: 10.1039/d4cp02492c Martin T Peschel 1 , Jörg Kussmann 1 , Christian Ochsenfeld 1, 2 , Regina de Vivie-Riedle 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-28 , DOI: 10.1039/d4cp02492c Martin T Peschel 1 , Jörg Kussmann 1 , Christian Ochsenfeld 1, 2 , Regina de Vivie-Riedle 1
Affiliation
|
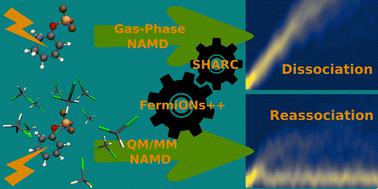
Unlocking the full potential of Lewis acid catalysis for photochemical transformations requires a comprehensive understanding of the ultrafast dynamics of substrate-Lewis acid complexes. In a previous article [Peschel et al., Angew. Chem. Int. Ed., 2021, 60, 10155], time-resolved spectroscopy supported by static calculations revealed that the Lewis acid remains attached during the relaxation of the model complex cyclohexenone-BF3. In contrast to the experimental observation, surface-hopping dynamics in the gas phase predicted ultrafast heterolytic dissociation. We attributed the discrepancy to missing solvent interactions. Thus, in this work, we present an interface between the SHARC and FermiONs++ program packages, which enables us to investigate the ultrafast dynamics of cyclohexenone-BF3 in an explicit solvent environment. Our simulations demonstrate that the solvent prevents the dissociation of the complex, leading to an intriguing dissociation–reassociation mechanism. Comparing the dynamics with and without triplet states highlights their role in the relaxation process and shows that the Lewis acid inhibits intersystem crossing. These findings provide a clear picture of the relaxation process, which may aid in designing future Lewis acid catalysts for photochemical applications. They underscore that an explicit solvent model is required to describe relaxation processes in weakly bound states, as energy transfer to the solvent is crucial for the system to reach its minimum geometries.
中文翻译:

烯酮-路易斯酸络合物在显式溶剂中的非绝热动力学模拟
要充分发挥路易斯酸催化光化学转化的潜力,需要全面了解底物-路易斯酸复合物的超快动力学。在之前的文章中 [Peschel等人,Angew。化学。国际。埃德。 , 2021, 60 , 10155],静态计算支持的时间分辨光谱表明,在模型复合物环己烯酮-BF 3的弛豫过程中,路易斯酸仍然保持附着状态。与实验观察相反,气相中的表面跳跃动力学预测了超快异解离解。我们将这种差异归因于缺少溶剂相互作用。因此,在这项工作中,我们提出了 SHARC 和 FermiONs++ 程序包之间的接口,这使我们能够研究环己烯酮-BF 3在显式溶剂环境中的超快动力学。我们的模拟表明,溶剂可以防止复合物解离,从而产生有趣的解离-重结合机制。比较有和没有三重态的动力学突出了它们在弛豫过程中的作用,并表明路易斯酸抑制系间窜越。这些发现提供了弛豫过程的清晰图像,这可能有助于设计未来用于光化学应用的路易斯酸催化剂。他们强调,需要一个显式溶剂模型来描述弱束缚态的弛豫过程,因为能量转移到溶剂对于系统达到其最小几何形状至关重要。
更新日期:2024-08-28
中文翻译:

烯酮-路易斯酸络合物在显式溶剂中的非绝热动力学模拟
要充分发挥路易斯酸催化光化学转化的潜力,需要全面了解底物-路易斯酸复合物的超快动力学。在之前的文章中 [Peschel等人,Angew。化学。国际。埃德。 , 2021, 60 , 10155],静态计算支持的时间分辨光谱表明,在模型复合物环己烯酮-BF 3的弛豫过程中,路易斯酸仍然保持附着状态。与实验观察相反,气相中的表面跳跃动力学预测了超快异解离解。我们将这种差异归因于缺少溶剂相互作用。因此,在这项工作中,我们提出了 SHARC 和 FermiONs++ 程序包之间的接口,这使我们能够研究环己烯酮-BF 3在显式溶剂环境中的超快动力学。我们的模拟表明,溶剂可以防止复合物解离,从而产生有趣的解离-重结合机制。比较有和没有三重态的动力学突出了它们在弛豫过程中的作用,并表明路易斯酸抑制系间窜越。这些发现提供了弛豫过程的清晰图像,这可能有助于设计未来用于光化学应用的路易斯酸催化剂。他们强调,需要一个显式溶剂模型来描述弱束缚态的弛豫过程,因为能量转移到溶剂对于系统达到其最小几何形状至关重要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号