当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Biochemical Insights into the Mechanism of Action of the Clinical USP1 Inhibitor, KSQ-4279
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-08-27 , DOI: 10.1021/acs.jmedchem.4c01184 Martin Luke Rennie 1 , Mehmet Gundogdu 2 , Connor Arkinson 1 , Steven Liness 2 , Sheelagh Frame 2 , Helen Walden 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-08-27 , DOI: 10.1021/acs.jmedchem.4c01184 Martin Luke Rennie 1 , Mehmet Gundogdu 2 , Connor Arkinson 1 , Steven Liness 2 , Sheelagh Frame 2 , Helen Walden 1
Affiliation
|
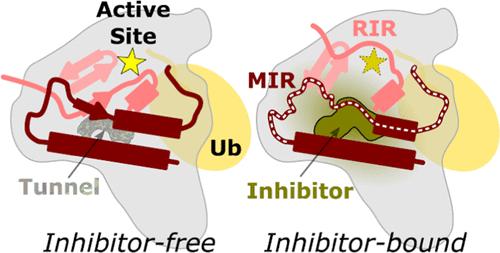
DNA damage triggers cell signaling cascades that mediate repair. This signaling is frequently dysregulated in cancers. The proteins that mediate this signaling are potential targets for therapeutic intervention. Ubiquitin-specific protease 1 (USP1) is one such target, with small-molecule inhibitors already in clinical trials. Here, we use biochemical assays and cryo-electron microscopy (cryo-EM) to study the clinical USP1 inhibitor, KSQ-4279 (RO7623066), and compare this to the well-established tool compound, ML323. We find that KSQ-4279 binds to the same cryptic site of USP1 as ML323 but disrupts the protein structure in subtly different ways. Inhibitor binding drives a substantial increase in thermal stability of USP1, which may be mediated through the inhibitors filling a hydrophobic tunnel-like pocket in USP1. Our results contribute to the understanding of the mechanism of action of USP1 inhibitors at the molecular level.
中文翻译:

对临床 USP1 抑制剂 KSQ-4279 作用机制的结构和生化见解
DNA 损伤会触发细胞信号级联反应,从而介导修复。这种信号传导在癌症中经常失调。介导这种信号传导的蛋白质是治疗干预的潜在目标。泛素特异性蛋白酶 1 (USP1) 就是这样的靶标之一,小分子抑制剂已进入临床试验。在这里,我们使用生化测定和冷冻电子显微镜 (cryo-EM) 研究临床 USP1 抑制剂 KSQ-4279 (RO7623066),并将其与成熟的工具化合物 ML323 进行比较。我们发现 KSQ-4279 与 ML323 结合到 USP1 的相同神秘位点,但以略有不同的方式破坏蛋白质结构。抑制剂结合可显着提高 USP1 的热稳定性,这可能是通过抑制剂填充 USP1 中的疏水隧道状口袋来介导的。我们的结果有助于在分子水平上理解 USP1 抑制剂的作用机制。
更新日期:2024-08-27
中文翻译:

对临床 USP1 抑制剂 KSQ-4279 作用机制的结构和生化见解
DNA 损伤会触发细胞信号级联反应,从而介导修复。这种信号传导在癌症中经常失调。介导这种信号传导的蛋白质是治疗干预的潜在目标。泛素特异性蛋白酶 1 (USP1) 就是这样的靶标之一,小分子抑制剂已进入临床试验。在这里,我们使用生化测定和冷冻电子显微镜 (cryo-EM) 研究临床 USP1 抑制剂 KSQ-4279 (RO7623066),并将其与成熟的工具化合物 ML323 进行比较。我们发现 KSQ-4279 与 ML323 结合到 USP1 的相同神秘位点,但以略有不同的方式破坏蛋白质结构。抑制剂结合可显着提高 USP1 的热稳定性,这可能是通过抑制剂填充 USP1 中的疏水隧道状口袋来介导的。我们的结果有助于在分子水平上理解 USP1 抑制剂的作用机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号