当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rotation and Self-Assembly Driving NLRP3 Activation
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-08-27 , DOI: 10.1021/acs.jcim.4c01112 Haochen Xu 1 , Zhonghuai Hou 1, 2 , Rongbin Zhou 3, 4 , Jie-Lou Liao 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-08-27 , DOI: 10.1021/acs.jcim.4c01112 Haochen Xu 1 , Zhonghuai Hou 1, 2 , Rongbin Zhou 3, 4 , Jie-Lou Liao 1
Affiliation
|
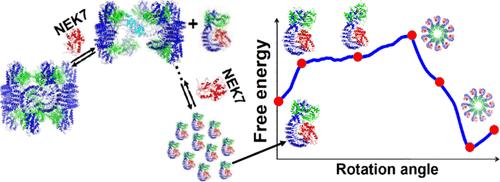
As a critical sensor protein, NLRP3 detects cellular perturbation caused by diverse exogenous and endogenous stimuli. NLRP3 activation requires domain rotation within the NEK7-bound NLRP3 monomer and assembly. However, a detailed molecular mechanism for NLRP3 assembly and activation remains elusive, particularly in terms of dynamics and energetics. In this work, all-atom molecular dynamics (MD) simulations are executed to describe large-amplitude closed-to-open conformational transitions along the rotational pathway. From the MD trajectories, the computed potential of mean force (PMF) shows that NLRP3 activation through monomeric domain rotation is an uphill process, during which the active conformation of the NLRP3-NEK7 monomer cannot be stabilized. Further binding free-energy calculations for two neighboring NLRP3-NEK7 subunits in a disc assembly with the C10 symmetry reveal that the protein self-assembly starts approximately at the 86.5° position on the rotary pathway, along which the NLRP3 activation becomes a downhill process to the active state at 90.5°. The active NLRP3-NEK7 monomeric conformation in the disc assembly is stabilized because of the interactions between the neighboring subunits, involving mainly FISNA loop 1 in one subunit and a “crocodile-clip” structure formed by the NBD helix-loop-strand motif (residues 351–373) and the WHD β-hairpin loop (residues 501–521) in the other. Our simulations also demonstrate that NEK7 plays an important role in the NLRP3 cage dissociation in the centrosome, which is consistent with biological experiments. The computational results provide kinetic, energetic, and structural insights into the molecular mechanisms of the activation of NLRP3 and the NEK7-driven dissociation of inactive NLRP3 cages. The activation mechanism of NLRP3 proposed in this work is significantly different from those of previous structural studies.
中文翻译:

旋转和自组装驱动 NLRP3 激活
作为一种关键的传感器蛋白,NLRP3 可检测由多种外源和内源刺激引起的细胞扰动。 NLRP3 激活需要 NEK7 结合的 NLRP3 单体和组装内的结构域旋转。然而,NLRP3 组装和激活的详细分子机制仍然难以捉摸,特别是在动力学和能量学方面。在这项工作中,执行全原子分子动力学(MD)模拟来描述沿着旋转路径的大振幅封闭到开放构象转变。从MD轨迹来看,平均力(PMF)的计算势表明,通过单体结构域旋转激活NLRP3是一个艰难的过程,在此期间NLRP3-NEK7单体的活性构象无法稳定。对具有 C10 对称性的圆盘组件中两个相邻 NLRP3-NEK7 亚基的进一步结合自由能计算表明,蛋白质自组装大约在旋转路径上的 86.5° 位置开始,沿着该路径,NLRP3 激活变成一个下坡过程90.5°处的活动状态。由于相邻亚基之间的相互作用,椎间盘组件中活性的NLRP3-NEK7单体构象得以稳定,主要涉及一个亚基中的FISNA环1和由NBD螺旋-环-链基序(残基)形成的“鳄鱼夹”结构351-373)和另一个中的 WHD β-发夹环(残基 501-521)。我们的模拟还表明,NEK7 在中心体中 NLRP3 笼解离中发挥重要作用,这与生物学实验一致。计算结果为 NLRP3 激活和 NEK7 驱动的非活性 NLRP3 笼解离的分子机制提供了动力学、能量和结构方面的见解。 本工作提出的NLRP3激活机制与之前的结构研究有显着不同。
更新日期:2024-08-30
中文翻译:

旋转和自组装驱动 NLRP3 激活
作为一种关键的传感器蛋白,NLRP3 可检测由多种外源和内源刺激引起的细胞扰动。 NLRP3 激活需要 NEK7 结合的 NLRP3 单体和组装内的结构域旋转。然而,NLRP3 组装和激活的详细分子机制仍然难以捉摸,特别是在动力学和能量学方面。在这项工作中,执行全原子分子动力学(MD)模拟来描述沿着旋转路径的大振幅封闭到开放构象转变。从MD轨迹来看,平均力(PMF)的计算势表明,通过单体结构域旋转激活NLRP3是一个艰难的过程,在此期间NLRP3-NEK7单体的活性构象无法稳定。对具有 C10 对称性的圆盘组件中两个相邻 NLRP3-NEK7 亚基的进一步结合自由能计算表明,蛋白质自组装大约在旋转路径上的 86.5° 位置开始,沿着该路径,NLRP3 激活变成一个下坡过程90.5°处的活动状态。由于相邻亚基之间的相互作用,椎间盘组件中活性的NLRP3-NEK7单体构象得以稳定,主要涉及一个亚基中的FISNA环1和由NBD螺旋-环-链基序(残基)形成的“鳄鱼夹”结构351-373)和另一个中的 WHD β-发夹环(残基 501-521)。我们的模拟还表明,NEK7 在中心体中 NLRP3 笼解离中发挥重要作用,这与生物学实验一致。计算结果为 NLRP3 激活和 NEK7 驱动的非活性 NLRP3 笼解离的分子机制提供了动力学、能量和结构方面的见解。 本工作提出的NLRP3激活机制与之前的结构研究有显着不同。































 京公网安备 11010802027423号
京公网安备 11010802027423号