当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interlayer-release synthesis of Cu single atoms anchored on a heterogeneous photocatalyst for constructing Cu–Ov–Fe bimetallic active sites with ultrafast kinetics of activation of H2O2
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-08-26 , DOI: 10.1039/d4ta04420g Fangfang Wang , Changdong Chen , Yan Shang , Shihui Shao , Wei Wang , Jiahao Zhu , Ying Gao , Lei Chen
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-08-26 , DOI: 10.1039/d4ta04420g Fangfang Wang , Changdong Chen , Yan Shang , Shihui Shao , Wei Wang , Jiahao Zhu , Ying Gao , Lei Chen
|
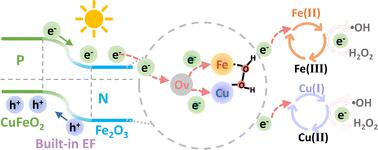
The efficient and selective generation of highly active ˙OH in a heterogeneous Fenton-like process is closely related to the spatial arrangement of active sites, local electron density of reactive atoms, and configuration of H2O2 adsorption to the active centers. Herein, a facile interlayer-release approach for the synthesis of a Cu single atom coordinated defect-rich Fe2O3/CuFeO2 heterogeneous photo-Fenton-like catalyst (Cu–Ov–Fe2O3/CuFeO2) with abundant atomically exposed Cu–Ov–Fe sites is developed. The asymmetric Cu–Ov–Fe sites, coupled with their acidic microenvironment, significantly enhance the adsorption of the Lewis-base H2O2 in a side-on configuration. This, in turn, promotes the homolytic cleavage of O–O bonds and selectively generates ˙OH. Fe2O3/CuFeO2 and agglomerated Cu clusters serve as the photosensitizers for efficient generation of photoexcited electrons, which are driven by a built-in electric field to the defect-rich Fe2O3 shell. Upon being trapped by the oxygen vacancies of Fe2O3, the electrons then flow directionally and get concentrated at the bimetallic Cu–Ov–Fe active sites, enhancing their electron density. The formation of the highly condensed localized electron density greatly improves the H2O2 activation kinetics. Benefitting from the excellent synergic effects of photocatalysis, single-atom catalysis, and asymmetric bimetallic activation effects, the well-designed Cu–Ov–Fe2O3/CuFeO2 exhibits record-breaking outstanding activity with TOF as high as 1.168 h−1 (99% degradation of phenol in 15 min), a remarkable selectivity of 78% for ˙OH, excellent durability with only 8% loss of activity after four runs and 23% loss after eight runs, and wide pH adaptability ranging from pH 4.5 to 9.
中文翻译:

锚定在异质光催化剂上的铜单原子的层间释放合成,用于构建具有超快 H2O2 活化动力学的 Cu-Ov-Fe 双金属活性位点
非均相类芬顿过程中高活性˙OH的高效选择性生成与活性位点的空间排列、反应原子的局部电子密度以及H 2 O 2吸附到活性中心的构型密切相关。在此,一种简便的层间释放方法用于合成Cu单原子配位的富含缺陷的Fe 2 O 3 /CuFeO 2非均相类光芬顿催化剂(Cu–O v –Fe 2 O 3 /CuFeO 2 ),该催化剂具有丰富的开发了原子暴露的 Cu-O v -Fe 位点。不对称的 Cu-O v- Fe 位点与其酸性微环境相结合,显着增强了侧向配置中路易斯碱 H 2 O 2的吸附。这反过来又促进 O-O 键的均裂并选择性地生成 ˙OH。 Fe 2 O 3 /CuFeO 2和团聚的Cu簇作为光敏剂,有效产生光生电子,这些电子由内置电场驱动到富含缺陷的Fe 2 O 3壳层。 一旦被Fe 2 O 3的氧空位捕获,电子就会定向流动并集中在双金属Cu-O v -Fe活性位点,从而增强其电子密度。高度凝聚的局域电子密度的形成极大地改善了H 2 O 2的活化动力学。受益于光催化、单原子催化和不对称双金属活化效应的优异协同效应,精心设计的Cu-O v -Fe 2 O 3 /CuFeO 2表现出破纪录的优异活性,TOF高达1.168 h - 1 (15 分钟内苯酚降解 99%),对 ˙OH 的选择性高达 78%,出色的耐久性,四次运行后活性仅损失 8%,八次运行后活性损失仅 23%,pH 值适应性广,范围为 pH 4.5到 9。
更新日期:2024-08-26
中文翻译:

锚定在异质光催化剂上的铜单原子的层间释放合成,用于构建具有超快 H2O2 活化动力学的 Cu-Ov-Fe 双金属活性位点
非均相类芬顿过程中高活性˙OH的高效选择性生成与活性位点的空间排列、反应原子的局部电子密度以及H 2 O 2吸附到活性中心的构型密切相关。在此,一种简便的层间释放方法用于合成Cu单原子配位的富含缺陷的Fe 2 O 3 /CuFeO 2非均相类光芬顿催化剂(Cu–O v –Fe 2 O 3 /CuFeO 2 ),该催化剂具有丰富的开发了原子暴露的 Cu-O v -Fe 位点。不对称的 Cu-O v- Fe 位点与其酸性微环境相结合,显着增强了侧向配置中路易斯碱 H 2 O 2的吸附。这反过来又促进 O-O 键的均裂并选择性地生成 ˙OH。 Fe 2 O 3 /CuFeO 2和团聚的Cu簇作为光敏剂,有效产生光生电子,这些电子由内置电场驱动到富含缺陷的Fe 2 O 3壳层。 一旦被Fe 2 O 3的氧空位捕获,电子就会定向流动并集中在双金属Cu-O v -Fe活性位点,从而增强其电子密度。高度凝聚的局域电子密度的形成极大地改善了H 2 O 2的活化动力学。受益于光催化、单原子催化和不对称双金属活化效应的优异协同效应,精心设计的Cu-O v -Fe 2 O 3 /CuFeO 2表现出破纪录的优异活性,TOF高达1.168 h - 1 (15 分钟内苯酚降解 99%),对 ˙OH 的选择性高达 78%,出色的耐久性,四次运行后活性仅损失 8%,八次运行后活性损失仅 23%,pH 值适应性广,范围为 pH 4.5到 9。





































 京公网安备 11010802027423号
京公网安备 11010802027423号