当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extracellular vesicle-derived miR-146a as a novel crosstalk mechanism for high-fat induced atherosclerosis by targeting SMAD4
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-08-08 , DOI: 10.1016/j.jare.2024.08.012 Kefeng Zhai 1 , Liangle Deng 2 , Yuxuan Wu 2 , Han Li 3 , Jing Zhou 3 , Ying Shi 3 , Jianhu Jia 2 , Wei Wang 4 , Sihui Nian 5 , Ghulam Jilany Khan 6 , Hesham R El-Seedi 7 , Hong Duan 3 , Lili Li 8 , Zhaojun Wei 9
中文翻译:

细胞外囊泡衍生的 miR-146a 作为靶向 SMAD4 治疗高脂肪诱导动脉粥样硬化的新型串扰机制
外泌体-miR-146a 在动脉粥样硬化 (AS) 患者中显著增加,但其机制和对 AS 的影响尚未完全阐明。
探讨外泌体释放的变化规律和机制,以及外泌体-miR-146a 在 AS 中的作用和分子机制。
我们分离并鉴定了用 ox-LDL 处理后的 THP-1 巨噬细胞的外泌体。然后使用免疫共沉淀和银染鉴定参与调节外泌体释放的蛋白质。PKH67 用于标记外泌体以确认细胞可以吸收它们,然后与 HVSMCs 共培养以进行细胞增殖和迁移检测。通过生物信息学和荧光素酶活性测定筛选鉴定 miR-146a 的靶基因,通过 qRT-PCR 和 Western blot 检测 HUVECs 中 miR-146a 和相关蛋白的表达。开发了高脂饮食诱导的 LDLR-/- 小鼠的 AS 模型,以研究外泌体 miR-146a 对 AS 的影响。
结果表明,来自 AS 的实验泡沫细胞显示 miR-146a 表达较高。据观察,NMMHC IIA 和 HSP70 相互作用调节外泌体的释放。HUVEC 可以吸收来自巨噬细胞的外泌体。此外,我们还发现 miR-146a 直接靶向 SMAD4 基因,调节 p38 MAPK 信号通路,从而介导 HUVECs 损伤。此外,外泌体 miR-146a 诱导 HVSMCs 的异常增殖和迁移。miR-146a-mimics 小鼠 miR-146a 的表达显著降低,miR-146a 抑制剂小鼠的表达升高,而 miR-146a 的抑制作用有效降低,而 miR-146a 的增加使小鼠的 AS 恶化。
我们的研究结果表明 miR-146a 有可能成为 AS 的有利治疗靶点,然而,进一步的探索有助于深入了解调节体内外泌体 miR-146a 释放的机制,并开发涉及 miR-146a 的有效治疗策略。
更新日期:2024-08-08
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-08-08 , DOI: 10.1016/j.jare.2024.08.012 Kefeng Zhai 1 , Liangle Deng 2 , Yuxuan Wu 2 , Han Li 3 , Jing Zhou 3 , Ying Shi 3 , Jianhu Jia 2 , Wei Wang 4 , Sihui Nian 5 , Ghulam Jilany Khan 6 , Hesham R El-Seedi 7 , Hong Duan 3 , Lili Li 8 , Zhaojun Wei 9
Affiliation
|
Introduction
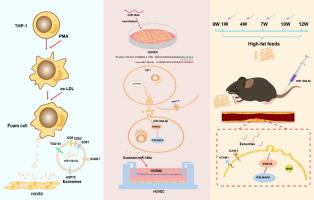
Exosome-miR-146a is significantly increased in patients with Atherosclerosis (AS), but its mechanism and effect on AS have not been fully elucidated.Objectives
To explore the change rule and mechanism of exosomes release, and the role and molecular mechanism of exosome-miR-146a in AS.Methods
We isolated and identified exosomes from THP-1 macrophages after treating them with ox-LDL. Then used co-immunoprecipitation and silver staining to identify the proteins involved in regulating exosome release. PKH67 was used to label exosomes to confirm that cells can absorb them, and then co-culture with HVSMCs for cell proliferation and migration detection. The target genes of miR-146a were screened and identified through bioinformatics and luciferase activity assay, and the expression of miR-146a and related proteins was detected through qRT-PCR and Western blot in HUVECs. An AS model in LDLR-/- mice induced by a high-fat diet was developed to investigate the impact of exosome-miR-146a on AS.Results
The results showed that experimental foam cells from AS showed higher expression of miR-146a. It was observed that NMMHC IIA and HSP70 interacted to regulate the release of exosomes. And HUVECs can absorb exosomes derived from macrophages. In addition, we also found that miR-146a directly targeted the SMAD4 gene to modulate the p38 MAPK signaling pathway, thereby mediating HUVECs damage. Furthermore, exosome-miR-146a induced abnormal proliferation and migration of HVSMCs. The expression of miR-146a was significantly reduced in miR-146a-mimics mice and increased in miR-146a inhibitor mice whereas the inhibition of miR-146a effectively reduced while increasing miR-146a worsened AS in mice.Conclusion
Our findings expressed the potential of miR-146a as a favorable therapeutic target for AS, however, further exploration is suggestive for deep understanding of the mechanisms regulating exosome-miR-146a release in vivo and to develop effective therapeutic strategies involving miR-146a.中文翻译:

细胞外囊泡衍生的 miR-146a 作为靶向 SMAD4 治疗高脂肪诱导动脉粥样硬化的新型串扰机制
介绍
外泌体-miR-146a 在动脉粥样硬化 (AS) 患者中显著增加,但其机制和对 AS 的影响尚未完全阐明。
目标
探讨外泌体释放的变化规律和机制,以及外泌体-miR-146a 在 AS 中的作用和分子机制。
方法
我们分离并鉴定了用 ox-LDL 处理后的 THP-1 巨噬细胞的外泌体。然后使用免疫共沉淀和银染鉴定参与调节外泌体释放的蛋白质。PKH67 用于标记外泌体以确认细胞可以吸收它们,然后与 HVSMCs 共培养以进行细胞增殖和迁移检测。通过生物信息学和荧光素酶活性测定筛选鉴定 miR-146a 的靶基因,通过 qRT-PCR 和 Western blot 检测 HUVECs 中 miR-146a 和相关蛋白的表达。开发了高脂饮食诱导的 LDLR-/- 小鼠的 AS 模型,以研究外泌体 miR-146a 对 AS 的影响。
结果
结果表明,来自 AS 的实验泡沫细胞显示 miR-146a 表达较高。据观察,NMMHC IIA 和 HSP70 相互作用调节外泌体的释放。HUVEC 可以吸收来自巨噬细胞的外泌体。此外,我们还发现 miR-146a 直接靶向 SMAD4 基因,调节 p38 MAPK 信号通路,从而介导 HUVECs 损伤。此外,外泌体 miR-146a 诱导 HVSMCs 的异常增殖和迁移。miR-146a-mimics 小鼠 miR-146a 的表达显著降低,miR-146a 抑制剂小鼠的表达升高,而 miR-146a 的抑制作用有效降低,而 miR-146a 的增加使小鼠的 AS 恶化。
结论
我们的研究结果表明 miR-146a 有可能成为 AS 的有利治疗靶点,然而,进一步的探索有助于深入了解调节体内外泌体 miR-146a 释放的机制,并开发涉及 miR-146a 的有效治疗策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号