当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multidose transient transfection of human embryonic kidney 293 cells modulates recombinant adeno-associated virus2/5 Rep protein expression and influences the enrichment fraction of filled capsids
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-08-23 , DOI: 10.1002/bit.28828 Prasanna Srinivasan 1 , Christopher T Canova 2 , Sha Sha 3 , Tam N T Nguyen 4 , John Joseph 1 , Jose Sangerman 1 , Andrew J Maloney 5 , Georgios Katsikis 6 , Rui Wen Ou 7 , Moo Sun Hong 8 , Jaclyn Ng 9 , Arella Yuan 1 , Daniel Antov 1 , Sally Song 1 , Wenyu Chen 1 , Caleb Neufeld 1 , Jacqueline M Wolfrum 1 , Paul W Barone 1 , Anthony J Sinskey 7 , Stacy L Springs 1 , Richard D Braatz 1, 2
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-08-23 , DOI: 10.1002/bit.28828 Prasanna Srinivasan 1 , Christopher T Canova 2 , Sha Sha 3 , Tam N T Nguyen 4 , John Joseph 1 , Jose Sangerman 1 , Andrew J Maloney 5 , Georgios Katsikis 6 , Rui Wen Ou 7 , Moo Sun Hong 8 , Jaclyn Ng 9 , Arella Yuan 1 , Daniel Antov 1 , Sally Song 1 , Wenyu Chen 1 , Caleb Neufeld 1 , Jacqueline M Wolfrum 1 , Paul W Barone 1 , Anthony J Sinskey 7 , Stacy L Springs 1 , Richard D Braatz 1, 2
Affiliation
|
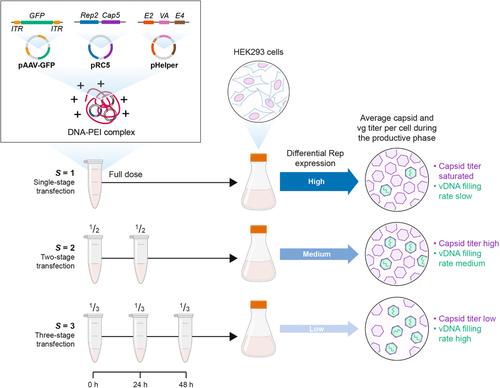
Recombinant adeno-associated virus (rAAV) is a commonly used in vivo gene therapy vector because of its nonpathogenicity, long-term transgene expression, broad tropism, and ability to transduce both dividing and nondividing cells. However, rAAV vector production via transient transfection of mammalian cells typically yields a low fraction of filled-to-total capsids (~1%–30% of total capsids produced). Analysis of our previously developed mechanistic model for rAAV2/5 production attributed these low fill fractions to a poorly coordinated timeline between capsid synthesis and viral DNA replication and the repression of later phase capsid formation by Rep proteins. Here, we extend the model by quantifying the expression dynamics of total Rep proteins and their influence on the key steps of rAAV2/5 production using a multiple dosing transfection of human embryonic kidney 293 (HEK293) cells. We report that the availability of preformed empty capsids and viral DNA copies per cell are not limiting to the capsid-filling reaction. However, optimal expression of Rep proteins (<240 ± 13 ag per cell) enables enrichment of the filled capsid population (>12% of total capsids/cell) upstream. Our analysis suggests increased enrichment of filled capsids via regulating the expression of Rep proteins is possible but at the expense of per cell capsid titer in a triple plasmid transfection. Our study reveals an intrinsic limitation of scaling rAAV2/5 vector genome (vg) production and underscores the need for approaches that allow for regulating the expression of Rep proteins to maximize vg titer per cell upstream.
中文翻译:

人胚胎肾 293 细胞的多剂量瞬时转染可调节重组腺相关病毒 2/5 Rep 蛋白表达并影响填充衣壳的富集分数
重组腺相关病毒 (rAAV) 是一种常用的体内基因治疗载体,因为它具有非致病性、长期转基因表达、广泛的嗜性以及转导分裂和非分裂细胞的能力。然而,通过哺乳动物细胞瞬时转染生产 rAAV 载体通常可产生低比例的填充至总衣壳(占产生的总衣壳的 ~1%–30%)。对我们之前开发的 rAAV2/5 产生机制模型的分析将这些低填充分数归因于衣壳合成和病毒 DNA 复制之间的时间线协调不良,以及 Rep 蛋白对后期衣壳形成的抑制。在这里,我们通过使用人胚胎肾 293 (HEK293) 细胞的多次给药转染量化总 Rep 蛋白的表达动力学及其对 rAAV2/5 产生关键步骤的影响来扩展模型。我们报道,每个细胞的预制空衣壳和病毒 DNA 拷贝的可用性并不局限于衣壳填充反应。然而,Rep 蛋白的最佳表达(<240 ± 13 ag/细胞)能够富集上游填充的衣壳群(占总衣壳/细胞的 >12%)。我们的分析表明,通过调节 Rep 蛋白的表达来增加填充衣壳的富集是可能的,但在三重质粒转染中以牺牲每个细胞的衣壳滴度为代价。我们的研究揭示了扩展 rAAV2/5 载体基因组 (vg) 产生的内在局限性,并强调了需要允许调节 Rep 蛋白表达的方法,以最大限度地提高上游每个细胞的 vg 滴度。
更新日期:2024-08-23
中文翻译:

人胚胎肾 293 细胞的多剂量瞬时转染可调节重组腺相关病毒 2/5 Rep 蛋白表达并影响填充衣壳的富集分数
重组腺相关病毒 (rAAV) 是一种常用的体内基因治疗载体,因为它具有非致病性、长期转基因表达、广泛的嗜性以及转导分裂和非分裂细胞的能力。然而,通过哺乳动物细胞瞬时转染生产 rAAV 载体通常可产生低比例的填充至总衣壳(占产生的总衣壳的 ~1%–30%)。对我们之前开发的 rAAV2/5 产生机制模型的分析将这些低填充分数归因于衣壳合成和病毒 DNA 复制之间的时间线协调不良,以及 Rep 蛋白对后期衣壳形成的抑制。在这里,我们通过使用人胚胎肾 293 (HEK293) 细胞的多次给药转染量化总 Rep 蛋白的表达动力学及其对 rAAV2/5 产生关键步骤的影响来扩展模型。我们报道,每个细胞的预制空衣壳和病毒 DNA 拷贝的可用性并不局限于衣壳填充反应。然而,Rep 蛋白的最佳表达(<240 ± 13 ag/细胞)能够富集上游填充的衣壳群(占总衣壳/细胞的 >12%)。我们的分析表明,通过调节 Rep 蛋白的表达来增加填充衣壳的富集是可能的,但在三重质粒转染中以牺牲每个细胞的衣壳滴度为代价。我们的研究揭示了扩展 rAAV2/5 载体基因组 (vg) 产生的内在局限性,并强调了需要允许调节 Rep 蛋白表达的方法,以最大限度地提高上游每个细胞的 vg 滴度。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号