当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Examining the Importance of Hydrogen Bonding and Proton Transfer in Iron Porphyrin-Mediated Carbon Dioxide Upconversion
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-20 , DOI: 10.1021/acs.accounts.4c00329 Jeffrey J Warren 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-20 , DOI: 10.1021/acs.accounts.4c00329 Jeffrey J Warren 1
Affiliation
|
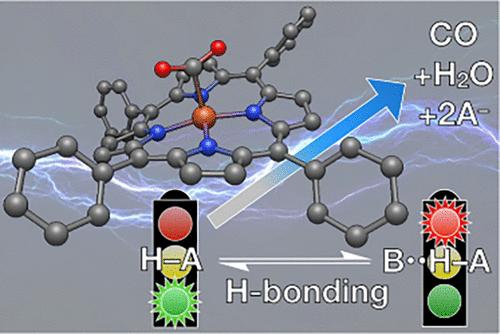
The title should give a sense of the “big picture” of this Account, but what is it really about? An unexpected change in research direction? A series of courageous and creative students? A team taking on challenging problems in chemistry? The answer is a definite “yes” to all of the above. More specifically, the problem in which we are interested is the upconversion or valorization of carbon dioxide. This problem has captured the attention of a great many chemists in earnest following the gas crisis of the 1970s and more recently galvanized due to climate concerns arising from the ongoing release of anthropogenic carbon. Addressing the problem of atmospheric carbon accumulation requires effort in two very broad areas: capture and conversion. Storage is an alternative to conversion, but this eliminates the opportunity to use what might be otherwise a waste product. Our group has investigated a series of modified versions of iron(III)–5,10,15,20-tetraphenylporphyrin (FeTPP) that can convert CO2 to carbon monoxide, which is a versatile and useful precursor for other syntheses. Following pioneering work from Savéant and his colleagues in the 1990s and thereafter, we started with a simple question: how many pendent ancillary groups that can donate H-bonds or protons are needed to support efficient CO2-to-CO conversion? Using a molecule with only one 2-hydroxylphenyl group, we demonstrated that the single prepositioned −OH group gave rise to efficient turnover, but only when experiments were carried out in a weakly H-bond-accepting solvent system. In other words, the ability of a solvent to accept H-bonds can impede CO2 reduction. We followed up with a deeper investigation of the influence of H-bonding interactions with external acids in FeTPP-mediated CO2 reduction. Savéant’s framework mechanism appears to be independent of solvent, and rate differences can be approximated by considering H-bonding equilibria. Following that work, we sought to better understand the minimum catalyst design requirements with respect to internal H-bond/proton donors. To that end, we produced all possible isomers of tetraarylpoprhyrins with 2,6-dihydroxyphenyl + phenyl groups. All else being equal, the complexes with a formally trans orientation of the 2,6-dihydroxyphenyl groups performed the best. Most recently, we surveyed the roles of internal and external Brønsted acids with different pKa values. Surprisingly, the best-performing catalysts have more weakly acidic internal groups. Overall, our work has demonstrated that CO2 reduction mediated by porphyrin catalysts can be improved by considering solvent H-bonding, the orientation of internal H-bonding groups, and the balance of the pKa values of internal and external acids. The future for molecular electrocatalysts is promising as more ideas emerge about how to design molecules and conditions for CO2 reduction.
中文翻译:

检查氢键和质子转移在铁卟啉介导的二氧化碳上转换中的重要性
标题应该让人了解这个帐户的“大局”,但它到底是关于什么的呢?研究方向发生意外变化?一系列勇敢而富有创造力的学生?一个团队正在解决化学方面的挑战性问题?对于上述所有问题,答案都是肯定的“是”。更具体地说,我们感兴趣的问题是二氧化碳的上转换或增值。继 20 世纪 70 年代的天然气危机之后,这个问题引起了许多化学家的认真关注,并且最近由于人为碳的持续释放引起的气候问题而更加严重。解决大气碳积累问题需要在两个非常广泛的领域做出努力:捕获和转化。存储是转换的替代方案,但这消除了使用本来可能是废品的机会。我们的小组研究了一系列铁(III)–5,10,15,20-四苯基卟啉(FeTPP)的改性版本,它们可以将CO 2转化为一氧化碳,一氧化碳是其他合成的多功能且有用的前体。继 Savéant 和他的同事在 20 世纪 90 年代及其后的开创性工作之后,我们从一个简单的问题开始:需要多少个可以提供氢键或质子的悬垂辅助基团来支持有效的 CO 2到 CO 的转化?使用仅具有一个 2-羟基苯基的分子,我们证明了单个前置的 -OH 基团可以产生有效的周转,但前提是实验是在弱氢键接受溶剂系统中进行的。换句话说,溶剂接受氢键的能力会阻碍CO 2还原。 我们随后对 FeTPP 介导的 CO 2还原中氢键与外部酸相互作用的影响进行了更深入的研究。 Savéant 的框架机制似乎与溶剂无关,并且可以通过考虑氢键平衡来近似速率差异。在这项工作之后,我们试图更好地了解内部氢键/质子供体的最低催化剂设计要求。为此,我们生产了带有 2,6-二羟基苯基 + 苯基的四芳基卟啉的所有可能的异构体。在其他条件相同的情况下,具有 2,6-二羟基苯基形式上反式取向的配合物表现最好。最近,我们调查了具有不同 p K a值的内部和外部布朗斯台德酸的作用。令人惊讶的是,性能最好的催化剂具有更多的弱酸性内部基团。总的来说,我们的工作表明,通过考虑溶剂氢键、内部氢键基团的方向以及内部和外部酸的p K a值的平衡,可以改善卟啉催化剂介导的CO 2还原。随着更多关于如何设计 CO 2还原分子和条件的想法不断涌现,分子电催化剂的未来充满希望。
更新日期:2024-08-20
中文翻译:

检查氢键和质子转移在铁卟啉介导的二氧化碳上转换中的重要性
标题应该让人了解这个帐户的“大局”,但它到底是关于什么的呢?研究方向发生意外变化?一系列勇敢而富有创造力的学生?一个团队正在解决化学方面的挑战性问题?对于上述所有问题,答案都是肯定的“是”。更具体地说,我们感兴趣的问题是二氧化碳的上转换或增值。继 20 世纪 70 年代的天然气危机之后,这个问题引起了许多化学家的认真关注,并且最近由于人为碳的持续释放引起的气候问题而更加严重。解决大气碳积累问题需要在两个非常广泛的领域做出努力:捕获和转化。存储是转换的替代方案,但这消除了使用本来可能是废品的机会。我们的小组研究了一系列铁(III)–5,10,15,20-四苯基卟啉(FeTPP)的改性版本,它们可以将CO 2转化为一氧化碳,一氧化碳是其他合成的多功能且有用的前体。继 Savéant 和他的同事在 20 世纪 90 年代及其后的开创性工作之后,我们从一个简单的问题开始:需要多少个可以提供氢键或质子的悬垂辅助基团来支持有效的 CO 2到 CO 的转化?使用仅具有一个 2-羟基苯基的分子,我们证明了单个前置的 -OH 基团可以产生有效的周转,但前提是实验是在弱氢键接受溶剂系统中进行的。换句话说,溶剂接受氢键的能力会阻碍CO 2还原。 我们随后对 FeTPP 介导的 CO 2还原中氢键与外部酸相互作用的影响进行了更深入的研究。 Savéant 的框架机制似乎与溶剂无关,并且可以通过考虑氢键平衡来近似速率差异。在这项工作之后,我们试图更好地了解内部氢键/质子供体的最低催化剂设计要求。为此,我们生产了带有 2,6-二羟基苯基 + 苯基的四芳基卟啉的所有可能的异构体。在其他条件相同的情况下,具有 2,6-二羟基苯基形式上反式取向的配合物表现最好。最近,我们调查了具有不同 p K a值的内部和外部布朗斯台德酸的作用。令人惊讶的是,性能最好的催化剂具有更多的弱酸性内部基团。总的来说,我们的工作表明,通过考虑溶剂氢键、内部氢键基团的方向以及内部和外部酸的p K a值的平衡,可以改善卟啉催化剂介导的CO 2还原。随着更多关于如何设计 CO 2还原分子和条件的想法不断涌现,分子电催化剂的未来充满希望。

































 京公网安备 11010802027423号
京公网安备 11010802027423号