当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Orthogonal Electrochemical Stability of Bulk and Surface in Lead Halide Perovskite Thin Films and Nanocrystals
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-08-23 , DOI: 10.1021/jacs.4c06340 Jence T. Mulder, Julius O. V. Monchen, Yan B. Vogel, Cheng Tai Lin, Filippo Drago, Valentina M. Caselli, Niranjan Saikumar, Tom J. Savenije, Arjan J. Houtepen
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-08-23 , DOI: 10.1021/jacs.4c06340 Jence T. Mulder, Julius O. V. Monchen, Yan B. Vogel, Cheng Tai Lin, Filippo Drago, Valentina M. Caselli, Niranjan Saikumar, Tom J. Savenije, Arjan J. Houtepen
|
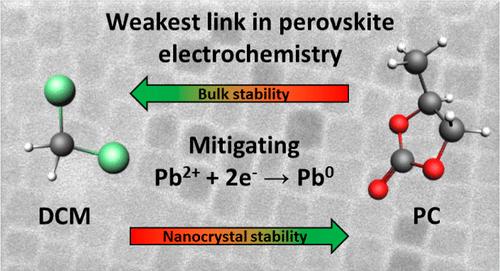
Lead halide perovskites have attracted significant attention for their wide-ranging applications in optoelectronic devices. A ubiquitous element in these applications is that charging of the perovskite is involved, which can trigger electrochemical degradation reactions. Understanding the underlying factors governing these degradation processes is crucial for improving the stability of perovskite-based devices. For bulk semiconductors, the electrochemical decomposition potentials depend on the stabilization of atoms in the lattice–a parameter linked to the material’s solubility. For perovskite nanocrystals (NCs), electrochemical surface reactions are strongly influenced by the binding equilibrium of passivating ligands. Here, we report a spectro-electrochemical study on CsPbBr3 NCs and bulk thin films in contact with various electrolytes, aimed at understanding the factors that control cathodic degradation. These measurements reveal that the cathodic decomposition of NCs is primarily determined by the solubility of surface ligands, with diminished cathodic degradation for NCs in high-polarity electrolyte solvents where ligand solubilities are lower. However, the solubility of the surface ligands and bulk lattice of NCs are orthogonal, such that no electrolyte could be identified where both the surface and bulk are stabilized against cathodic decomposition. This poses inherent challenges for electrochemical applications: (i) The electrochemical stability window of CsPbBr3 NCs is constrained by the reduction potential of dissolved Pb2+ complexes, and (ii) cathodic decomposition occurs well before the conduction band can be populated with electrons. Our findings provide insights to enhance the electrochemical stability of perovskite thin films and NCs, emphasizing the importance of a combined selection of surface passivation and electrolyte.
中文翻译:

卤化铅钙钛矿薄膜和纳米晶体体和表面的正交电化学稳定性
卤化铅钙钛矿因其在光电器件中的广泛应用而引起了人们的广泛关注。这些应用中普遍存在的一个因素是钙钛矿的充电,这会引发电化学降解反应。了解控制这些降解过程的根本因素对于提高钙钛矿器件的稳定性至关重要。对于块状半导体,电化学分解电势取决于晶格中原子的稳定性——一个与材料溶解度相关的参数。对于钙钛矿纳米晶体(NC),电化学表面反应受到钝化配体的结合平衡的强烈影响。在这里,我们报告了一项对 CsPbBr 3 NC 和块状薄膜与各种电解质接触的光谱电化学研究,旨在了解控制阴极降解的因素。这些测量结果表明,NC 的阴极分解主要由表面配体的溶解度决定,在配体溶解度较低的高极性电解质溶剂中,NC 的阴极降解会减弱。然而,表面配体的溶解度和NC的体晶格是正交的,因此在表面和体都稳定以防止阴极分解的情况下无法识别电解质。这给电化学应用带来了固有的挑战:(i) CsPbBr 3 NCs 的电化学稳定性窗口受到溶解的 Pb 2+络合物的还原电位的限制,(ii) 阴极分解发生在导带充满电子之前。 我们的研究结果为增强钙钛矿薄膜和纳米晶的电化学稳定性提供了见解,强调了表面钝化和电解质组合选择的重要性。
更新日期:2024-08-23
中文翻译:

卤化铅钙钛矿薄膜和纳米晶体体和表面的正交电化学稳定性
卤化铅钙钛矿因其在光电器件中的广泛应用而引起了人们的广泛关注。这些应用中普遍存在的一个因素是钙钛矿的充电,这会引发电化学降解反应。了解控制这些降解过程的根本因素对于提高钙钛矿器件的稳定性至关重要。对于块状半导体,电化学分解电势取决于晶格中原子的稳定性——一个与材料溶解度相关的参数。对于钙钛矿纳米晶体(NC),电化学表面反应受到钝化配体的结合平衡的强烈影响。在这里,我们报告了一项对 CsPbBr 3 NC 和块状薄膜与各种电解质接触的光谱电化学研究,旨在了解控制阴极降解的因素。这些测量结果表明,NC 的阴极分解主要由表面配体的溶解度决定,在配体溶解度较低的高极性电解质溶剂中,NC 的阴极降解会减弱。然而,表面配体的溶解度和NC的体晶格是正交的,因此在表面和体都稳定以防止阴极分解的情况下无法识别电解质。这给电化学应用带来了固有的挑战:(i) CsPbBr 3 NCs 的电化学稳定性窗口受到溶解的 Pb 2+络合物的还原电位的限制,(ii) 阴极分解发生在导带充满电子之前。 我们的研究结果为增强钙钛矿薄膜和纳米晶的电化学稳定性提供了见解,强调了表面钝化和电解质组合选择的重要性。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号