当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single-Molecule Characterization of Heterogeneous Oligomer Formation during Co-Aggregation of 40- and 42-Residue Amyloid-β
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-08-23 , DOI: 10.1021/jacs.4c06372 Fanjie Meng, Jae-Yeol Kim, John M. Louis, Hoi Sung Chung
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-08-23 , DOI: 10.1021/jacs.4c06372 Fanjie Meng, Jae-Yeol Kim, John M. Louis, Hoi Sung Chung
|
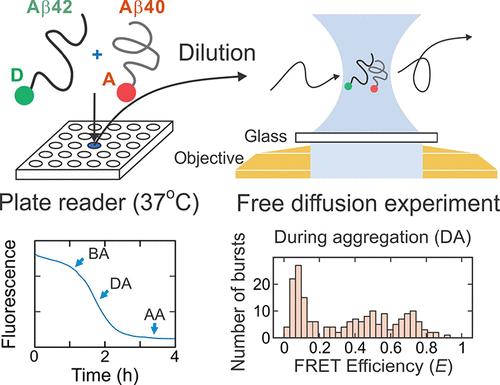
The two most abundant isoforms of amyloid-β (Aβ) are the 40- (Aβ40) and 42-residue (Aβ42) peptides. Since they coexist and there is a correlation between toxicity and the ratio of the two isoforms, quantitative characterization of their interactions is crucial for understanding the Aβ aggregation mechanism. In this work, we follow the aggregation of individual isoforms in a mixture using single-molecule FRET spectroscopy by labeling Aβ42 and Aβ40 with the donor and acceptor fluorophores, respectively. We found that there are two phases of aggregation. The first phase consists of coaggregation of Aβ42 with a small amount of Aβ40, while the second phase results mostly from aggregation of Aβ40. We also found that the aggregation of Aβ42 is slowed by Aβ40 while the aggregation of Aβ40 is accelerated by Aβ42 in a concentration-dependent manner. The formation of oligomers was monitored by incubating mixtures in a plate reader and performing a single-molecule free-diffusion experiment at several different stages of aggregation. The detailed properties of the oligomers were obtained by maximum likelihood analysis of fluorescence bursts. The FRET efficiency distribution is much broader than that of the Aβ42 oligomers, indicating the diversity in isoform composition of the oligomers. Pulsed interleaved excitation experiments estimate that the fraction of Aβ40 in the co-oligomers in a 1:1 mixture of Aβ42 and Aβ40 varies between 0 and 20%. The detected oligomers were mostly co-oligomers especially at the physiological ratio of Aβ42 and Aβ40 (1:10), suggesting the critical role of Aβ40 in oligomer formation and aggregation.
中文翻译:

40 和 42 残基淀粉样蛋白-β 共聚集过程中异质寡聚物形成的单分子表征
β 淀粉样蛋白 (Aβ) 的两种最丰富的亚型是 40 残基 (Aβ40) 和 42 残基 (Aβ42) 肽。由于它们共存,并且毒性与两种亚型的比例之间存在相关性,因此它们相互作用的定量表征对于理解 Aβ 聚集机制至关重要。在这项工作中,我们使用单分子 FRET 光谱分别用供体和受体荧光团标记 Aβ42 和 Aβ40,跟踪混合物中各个异构体的聚集。我们发现聚合有两个阶段。第一相由 Aβ42 与少量 Aβ40 共聚集组成,而第二相主要由 Aβ40 聚集形成。我们还发现,Aβ40 会减慢 Aβ42 的聚集,而 Aβ42 会以浓度依赖性方式加速 Aβ40 的聚集。通过在读板器中孵育混合物并在几个不同的聚集阶段进行单分子自由扩散实验来监测低聚物的形成。通过荧光爆发的最大似然分析获得了低聚物的详细特性。 FRET 效率分布比 Aβ42 寡聚体的 FRET 效率分布要宽得多,表明寡聚体异构体组成的多样性。脉冲交错激发实验估计,Aβ42 和 Aβ40 的 1:1 混合物中的共聚低聚物中的 Aβ40 分数在 0 至 20% 之间变化。检测到的寡聚物主要是共寡聚物,特别是在 Aβ42 和 Aβ40 的生理比例 (1:10) 下,表明 Aβ40 在寡聚物形成和聚集中起着关键作用。
更新日期:2024-08-23
中文翻译:

40 和 42 残基淀粉样蛋白-β 共聚集过程中异质寡聚物形成的单分子表征
β 淀粉样蛋白 (Aβ) 的两种最丰富的亚型是 40 残基 (Aβ40) 和 42 残基 (Aβ42) 肽。由于它们共存,并且毒性与两种亚型的比例之间存在相关性,因此它们相互作用的定量表征对于理解 Aβ 聚集机制至关重要。在这项工作中,我们使用单分子 FRET 光谱分别用供体和受体荧光团标记 Aβ42 和 Aβ40,跟踪混合物中各个异构体的聚集。我们发现聚合有两个阶段。第一相由 Aβ42 与少量 Aβ40 共聚集组成,而第二相主要由 Aβ40 聚集形成。我们还发现,Aβ40 会减慢 Aβ42 的聚集,而 Aβ42 会以浓度依赖性方式加速 Aβ40 的聚集。通过在读板器中孵育混合物并在几个不同的聚集阶段进行单分子自由扩散实验来监测低聚物的形成。通过荧光爆发的最大似然分析获得了低聚物的详细特性。 FRET 效率分布比 Aβ42 寡聚体的 FRET 效率分布要宽得多,表明寡聚体异构体组成的多样性。脉冲交错激发实验估计,Aβ42 和 Aβ40 的 1:1 混合物中的共聚低聚物中的 Aβ40 分数在 0 至 20% 之间变化。检测到的寡聚物主要是共寡聚物,特别是在 Aβ42 和 Aβ40 的生理比例 (1:10) 下,表明 Aβ40 在寡聚物形成和聚集中起着关键作用。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号