当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N2O Decomposition on Singly and Doubly (K and Li)-Doped Co3O4 Nanocubes─Establishing Key Factors Governing Redox Behavior of Catalysts
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-08-23 , DOI: 10.1021/jacs.4c06587 Leszek Nowakowski, Camillo Hudy, Filip Zasada, Joanna Gryboś, Witold Piskorz, Anna Wach, Yves Kayser, Jakub Szlachetko, Zbigniew Sojka
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-08-23 , DOI: 10.1021/jacs.4c06587 Leszek Nowakowski, Camillo Hudy, Filip Zasada, Joanna Gryboś, Witold Piskorz, Anna Wach, Yves Kayser, Jakub Szlachetko, Zbigniew Sojka
|
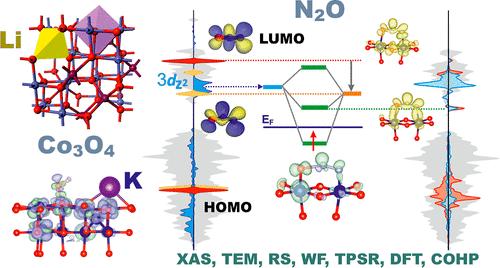
The intimate mechanism of N2O decomposition on bare and redox-tuned Co3O4 nanocubes (achieved by single (Li or K) and double (Li and K) doping) was elucidated. The catalysts synthesized by the hydrothermal method were characterized by X-ray electron absorption fine structure measurements, X-ray diffraction, Raman spectroscopy, scanning electron microscopy, transmission electron microscopy, and Kelvin Probe techniques. TPSR and steady-state isothermal catalytic tests reveal that the N2O turnover frequencies are critically sensitive to the work function of the catalysts, adjusted purposely by doping. For the catalysts obtained by one-pot hydrothermal synthesis, lithiation of the Co3O4 nanocubes leads to the formation of {Li’8a, Co·16d} species, decreasing steadily the work function and the activity, while for the catalysts prepared by postsynthesis impregnation, formation of {Li’8a, Co’16d, Co··16c} species leads to a volcano-type dependence of the catalytic activity and the work function in parallel. The beneficial effect of potassium was discussed in terms of mitigation of surface potential buildup due to the accumulation of ionosorbed oxygen intermediates (surface electrostatics), which hinders the interfacial electron transfer. Analysis of the catalytic activity response to the redox tuning of Co3O4, substantiated by DFT calculations, allowed for a straightforward conceptualization of the redox nature of the N2O decomposition in terms of the lineup of frontier orbitals of the N2O/N2O– and O2–/O2 reactants with the surface DOS structure and the resultant molecular orbital interactions. The positions of the virtual bonding 3πg0(N2O)−α-3dz2 and the occupied 2πg1(O2–)−α-3dz2 states relative to the Fermi energy level play a crucial role in the regulation of the forward and backward interfacial electron transfer events, which drive the redox process.
中文翻译:

单、双(钾和锂)掺杂 Co3O4 纳米立方体上的 N2O 分解─建立控制催化剂氧化还原行为的关键因素
阐明了裸露和氧化还原调节的Co 3 O 4纳米立方体(通过单(Li或K)和双(Li和K)掺杂实现)上N 2 O分解的密切机制。通过X射线电子吸收精细结构测量、X射线衍射、拉曼光谱、扫描电子显微镜、透射电子显微镜和开尔文探针技术对水热法合成的催化剂进行了表征。 TPSR和稳态等温催化测试表明,N 2 O转换频率对催化剂的功函数非常敏感,并通过掺杂进行有目的地调整。对于一锅水热合成得到的催化剂,Co 3 O 4纳米立方体的锂化导致{Li' 8a , Co· 16d }物种的形成,使功函数和活性稳步下降,而对于通过合成后浸渍,{Li' 8a , Co' 16d, Co·· 16c }物质的形成导致催化活性和功函数并行的火山型依赖性。钾的有益作用是在减轻由于离子吸附氧中间体(表面静电)的积累而阻碍界面电子转移而导致的表面电位积累方面进行讨论的。 通过 DFT 计算证实,对 Co 3 O 4氧化还原调节的催化活性响应分析允许根据 N 2 O/ 前沿轨道的排列对 N 2 O 分解的氧化还原性质进行简单的概念化N 2 O –和O 2 – /O 2反应物具有表面DOS结构以及由此产生的分子轨道相互作用。虚键 3π g 0 (N 2 O)−α-3d z 2和占据的 2π g 1 (O 2 – )−α-3d z 2态相对于费米能级的位置在调节驱动氧化还原过程的向前和向后界面电子转移事件。
更新日期:2024-08-23
中文翻译:

单、双(钾和锂)掺杂 Co3O4 纳米立方体上的 N2O 分解─建立控制催化剂氧化还原行为的关键因素
阐明了裸露和氧化还原调节的Co 3 O 4纳米立方体(通过单(Li或K)和双(Li和K)掺杂实现)上N 2 O分解的密切机制。通过X射线电子吸收精细结构测量、X射线衍射、拉曼光谱、扫描电子显微镜、透射电子显微镜和开尔文探针技术对水热法合成的催化剂进行了表征。 TPSR和稳态等温催化测试表明,N 2 O转换频率对催化剂的功函数非常敏感,并通过掺杂进行有目的地调整。对于一锅水热合成得到的催化剂,Co 3 O 4纳米立方体的锂化导致{Li' 8a , Co· 16d }物种的形成,使功函数和活性稳步下降,而对于通过合成后浸渍,{Li' 8a , Co' 16d, Co·· 16c }物质的形成导致催化活性和功函数并行的火山型依赖性。钾的有益作用是在减轻由于离子吸附氧中间体(表面静电)的积累而阻碍界面电子转移而导致的表面电位积累方面进行讨论的。 通过 DFT 计算证实,对 Co 3 O 4氧化还原调节的催化活性响应分析允许根据 N 2 O/ 前沿轨道的排列对 N 2 O 分解的氧化还原性质进行简单的概念化N 2 O –和O 2 – /O 2反应物具有表面DOS结构以及由此产生的分子轨道相互作用。虚键 3π g 0 (N 2 O)−α-3d z 2和占据的 2π g 1 (O 2 – )−α-3d z 2态相对于费米能级的位置在调节驱动氧化还原过程的向前和向后界面电子转移事件。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号