当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
C3 Selective Hydroxylation of Pyridines via Photochemical Valence Isomerization of Pyridine N-Oxides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-08-22 , DOI: 10.1021/jacs.4c10057 Chen-Yan Cai 1 , Si-Jie Chen 2 , Rohan R. Merchant 2 , Yuzuru Kanda 3 , Tian Qin 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-08-22 , DOI: 10.1021/jacs.4c10057 Chen-Yan Cai 1 , Si-Jie Chen 2 , Rohan R. Merchant 2 , Yuzuru Kanda 3 , Tian Qin 1
Affiliation
|
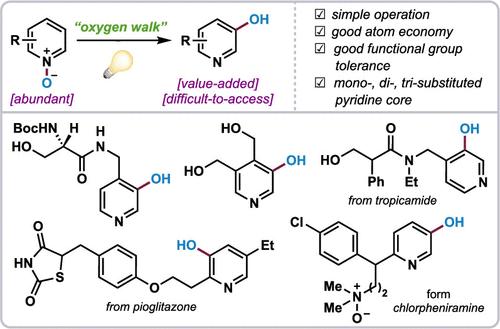
The C–H hydroxylation of the pyridine C3 position is a highly desirable transformation but remains a great challenge due to the inherent electronic properties of this heterocycle core which bring difficulties in chemical reactivity and regioselectivity. Herein we present an efficient method for formal C3 selective hydroxylation of pyridines via photochemical valence isomerization of pyridine N-oxides. This metal-free transformation features operational simplicity and compatibility with a diverse array of functional groups, and the resulting hydroxylated products are amenable to further elaboration to synthetically useful building blocks. The synthetic utility of this strategy is further demonstrated in the effective late-stage functionalization of pyridine-containing medicinally relevant molecules and versatile derivatizations of 3-pyridinols.
中文翻译:

通过吡啶 N-氧化物的光化学价异构化对吡啶进行 C3 选择性羟基化
吡啶C3位的C-H羟基化是一种非常理想的转化,但由于该杂环核固有的电子特性给化学反应性和区域选择性带来了困难,因此仍然是一个巨大的挑战。在此,我们提出了一种通过吡啶N-氧化物的光化学价异构化对吡啶进行形式 C3 选择性羟基化的有效方法。这种无金属转化的特点是操作简单且与多种官能团兼容,所得羟基化产物适合进一步精制为合成有用的结构单元。该策略的合成效用在含吡啶医学相关分子的有效后期功能化和 3-吡啶醇的多功能衍生化中得到了进一步证明。
更新日期:2024-08-23
中文翻译:

通过吡啶 N-氧化物的光化学价异构化对吡啶进行 C3 选择性羟基化
吡啶C3位的C-H羟基化是一种非常理想的转化,但由于该杂环核固有的电子特性给化学反应性和区域选择性带来了困难,因此仍然是一个巨大的挑战。在此,我们提出了一种通过吡啶N-氧化物的光化学价异构化对吡啶进行形式 C3 选择性羟基化的有效方法。这种无金属转化的特点是操作简单且与多种官能团兼容,所得羟基化产物适合进一步精制为合成有用的结构单元。该策略的合成效用在含吡啶医学相关分子的有效后期功能化和 3-吡啶醇的多功能衍生化中得到了进一步证明。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号