当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
84.0% energy-efficient nitrate conversion by a defective (Fe,Cu,Ni)2O3 electrocatalyst
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-08-23 , DOI: 10.1039/d4ta02801e Tadele Negash Gemeda, Dong-Hau Kuo, Quoc-Nam Ha, Noto Susanto Gultom, Girma Sisay Wolde
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-08-23 , DOI: 10.1039/d4ta02801e Tadele Negash Gemeda, Dong-Hau Kuo, Quoc-Nam Ha, Noto Susanto Gultom, Girma Sisay Wolde
|
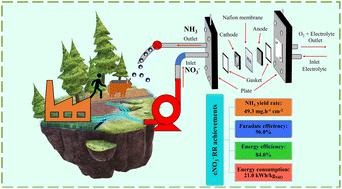
Decentralized ammonia (NH3) production as a way of environmental remediation for nitrate (NO3−) removal is a current issue due to the massive impact of nitrate on human well-being and the environment. Converting these contaminating NO3− species to valuable commodities such as NH3 in a green and sustainable way with a high NO3− conversion rate at low energy consumption efficiency can help to protect the environment and improve the livelihood of human beings. We synthesized an in situ grown and nanoflower-type (Fe,Cu,Ni)2O3−x electrocatalytic active material via a facile hydrothermal method. This catalyst exhibited a high NO3− conversion of 92.6% into a dominant ammonia with a selectivity of 98.3%. The NH3 was produced at a high yield rate of 9.2 mg h−1 cm−2 with faradaic efficiency (FE) of 94.8% at −0.3 V vs. the reversible hydrogen electrode (RHE) in an H-cell configuration. For a viable commercialization test, the scaled-up model of a single-stack flow cell electrolyzer was investigated and it accelerated the NH3 yield rate to 49.3 mg h−1 cm−2 and 96.0% FE while holding a high energy efficiency of 84.0% and a low energy consumption of 21.0 kW h kg−1. The NH3 yield exceeds that of the current state-of-the-art due to the reactants' continuous and high mass transfer rate on the trimetallic oxide active sites. The elucidated electrokinetic reaction mechanism reveals the direct reduction pathway enhanced by the abundant oxygen-vacancy (OV) sites in a synergetic trimetallic oxide system. These OV sites play a crucial role in the reaction mechanism by facilitating the trapping of oxygen atoms in NO3−, which are then surrounded by Fe2+/3+, Cu+, and Ni2+/3+ as sites of proton adsorption for NO3− hydrogenation. The trapping interaction enhancing the bond weakening of NO3− makes the electrochemical NO3− reduction reaction (eNO3−RR) mechanism meaningful.
中文翻译:

通过有缺陷的 (Fe,Cu,Ni)2O3 电催化剂实现 84.0% 能效的硝酸盐转化
由于硝酸盐对人类福祉和环境的巨大影响,分散式氨(NH 3 )生产作为去除硝酸盐(NO 3 - )的环境修复方式是当前的一个问题。以绿色、可持续的方式,以低能耗效率、高NO 3 -转化率,将这些污染性NO 3 -物质转化为NH 3等有价值的商品,有助于保护环境、改善人类生计。我们通过简单的水热方法合成了原位生长的纳米花型(Fe,Cu,Ni) 2 O 3− x电催化活性材料。该催化剂表现出将92.6%的NO 3 -转化为主要氨的高转化率,选择性为98.3%。与 H 电池配置中的可逆氢电极 (RHE)相比,在 -0.3 V 下,NH 3的产率高达 9.2 mg h -1 cm -2 ,法拉第效率 (FE) 为 94.8%。为了进行可行的商业化测试,研究了单堆流通池电解槽的放大模型,并将 NH 3产率加快至 49。3 mg h -1 cm -2和96.0% FE,同时保持84.0%的高能效和21.0 kW h kg -1的低能耗。由于反应物在三金属氧化物活性位点上的连续且高的传质速率,NH 3产率超过了当前最先进的水平。阐明的动电反应机制揭示了协同三金属氧化物系统中丰富的氧空位(OV)位点增强的直接还原途径。这些 OV 位点在反应机理中发挥着至关重要的作用,有助于捕获 NO 3 -中的氧原子,然后被 Fe 2+/3+ 、Cu +和 Ni 2+/3+包围作为质子吸附位点用于NO 3 −氢化。捕获相互作用增强了NO 3 -的键弱化,使得电化学NO 3 -还原反应(eNO 3 - RR)机制变得有意义。
更新日期:2024-08-23
中文翻译:

通过有缺陷的 (Fe,Cu,Ni)2O3 电催化剂实现 84.0% 能效的硝酸盐转化
由于硝酸盐对人类福祉和环境的巨大影响,分散式氨(NH 3 )生产作为去除硝酸盐(NO 3 - )的环境修复方式是当前的一个问题。以绿色、可持续的方式,以低能耗效率、高NO 3 -转化率,将这些污染性NO 3 -物质转化为NH 3等有价值的商品,有助于保护环境、改善人类生计。我们通过简单的水热方法合成了原位生长的纳米花型(Fe,Cu,Ni) 2 O 3− x电催化活性材料。该催化剂表现出将92.6%的NO 3 -转化为主要氨的高转化率,选择性为98.3%。与 H 电池配置中的可逆氢电极 (RHE)相比,在 -0.3 V 下,NH 3的产率高达 9.2 mg h -1 cm -2 ,法拉第效率 (FE) 为 94.8%。为了进行可行的商业化测试,研究了单堆流通池电解槽的放大模型,并将 NH 3产率加快至 49。3 mg h -1 cm -2和96.0% FE,同时保持84.0%的高能效和21.0 kW h kg -1的低能耗。由于反应物在三金属氧化物活性位点上的连续且高的传质速率,NH 3产率超过了当前最先进的水平。阐明的动电反应机制揭示了协同三金属氧化物系统中丰富的氧空位(OV)位点增强的直接还原途径。这些 OV 位点在反应机理中发挥着至关重要的作用,有助于捕获 NO 3 -中的氧原子,然后被 Fe 2+/3+ 、Cu +和 Ni 2+/3+包围作为质子吸附位点用于NO 3 −氢化。捕获相互作用增强了NO 3 -的键弱化,使得电化学NO 3 -还原反应(eNO 3 - RR)机制变得有意义。





































 京公网安备 11010802027423号
京公网安备 11010802027423号