当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Differentiating ion transport of water-in-salt electrolytes within micro- and meso-pores of a multiporous carbon electrode
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-08-22 , DOI: 10.1039/d4ta03632h M. Tauhidul Islam 1 , Bernhard Gollas 1 , Qamar Abbas 1, 2
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-08-22 , DOI: 10.1039/d4ta03632h M. Tauhidul Islam 1 , Bernhard Gollas 1 , Qamar Abbas 1, 2
Affiliation
|
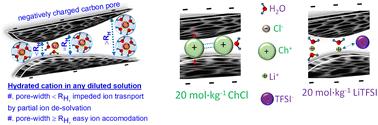
Understanding ion transport in porous carbon electrodes is crucial for enhancing the performance of electrochemical energy storage devices. However, for systems using carbon electrodes and water-in-salt electrolytes, this is not generally understood. Here, two salts with different ionic interactions in water, lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) or choline chloride (ChCl), were utilized at concentrations up to 20 mol kg−1 to explore the ion transport behavior. We report a new method for calculating the ion diffusion coefficient in carbon pores, considering the diffusivity of the bulk electrolyte, as well as the tortuosity and porosity of the carbon electrode. Accuracy is validated by comparing data with bulk electrolyte diffusivity reported from PFG-NMR in the literature, which is further used together with porosity estimated with nitrogen gas adsorption and tortuosity from electrochemical impedance spectroscopy. This technique effectively distinguishes between tortuosity in micro- and meso-pores by considering their volume and surface area. The different ion hydration patterns of ChCl and LiTFSI at concentrations above 10 mol kg−1 influence the ion transport and the tortuosity to different extents. This is confirmed by changes in hydrogen bonding observed in the Raman water bands. Lastly, we introduce a relationship between tortuosity, in-pore ion diffusivity and capacitance to distinguish the charge distribution within micro- and meso-pores at open circuit voltage as well as under applied bias voltages. Our findings reveal the degree of ion dissociation in concentrated aqueous electrolytes as a key parameter determining the charging/discharging rate performance of carbon electrode based capacitors. This study helps develop carbon materials and compatible electrolytes to ensure that the capacitor meets the desired performance criteria while being reliable and efficient.
中文翻译:

多孔碳电极微孔和中孔内盐包水电解质的离子传输的差异
了解多孔碳电极中的离子传输对于提高电化学储能装置的性能至关重要。然而,对于使用碳电极和盐包水电解质的系统,人们普遍不理解这一点。在这里,使用浓度高达 20 mol kg -1 的两种在水中具有不同离子相互作用的盐,双(三氟甲磺酰基)亚胺锂(LiTFSI)或氯化胆碱(ChCl)来探索离子传输行为。我们报告了一种计算碳孔中离子扩散系数的新方法,考虑了本体电解质的扩散率以及碳电极的弯曲度和孔隙率。通过将数据与文献中 PFG-NMR 报告的本体电解质扩散率进行比较来验证准确性,该数据进一步与通过氮气吸附估计的孔隙率和电化学阻抗谱的弯曲度一起使用。该技术通过考虑微孔和中孔的体积和表面积,有效地区分微孔和中孔的弯曲度。 ChCl和LiTFSI在浓度高于10 mol kg -1时的不同离子水合模式不同程度地影响离子传输和弯曲度。在拉曼水带中观察到的氢键变化证实了这一点。最后,我们引入了弯曲度、孔内离子扩散率和电容之间的关系,以区分开路电压以及施加偏置电压下微孔和中孔内的电荷分布。 我们的研究结果表明,浓水电解质中的离子解离程度是决定碳电极电容器充电/放电速率性能的关键参数。这项研究有助于开发碳材料和兼容的电解质,以确保电容器满足所需的性能标准,同时可靠和高效。
更新日期:2024-08-22
中文翻译:

多孔碳电极微孔和中孔内盐包水电解质的离子传输的差异
了解多孔碳电极中的离子传输对于提高电化学储能装置的性能至关重要。然而,对于使用碳电极和盐包水电解质的系统,人们普遍不理解这一点。在这里,使用浓度高达 20 mol kg -1 的两种在水中具有不同离子相互作用的盐,双(三氟甲磺酰基)亚胺锂(LiTFSI)或氯化胆碱(ChCl)来探索离子传输行为。我们报告了一种计算碳孔中离子扩散系数的新方法,考虑了本体电解质的扩散率以及碳电极的弯曲度和孔隙率。通过将数据与文献中 PFG-NMR 报告的本体电解质扩散率进行比较来验证准确性,该数据进一步与通过氮气吸附估计的孔隙率和电化学阻抗谱的弯曲度一起使用。该技术通过考虑微孔和中孔的体积和表面积,有效地区分微孔和中孔的弯曲度。 ChCl和LiTFSI在浓度高于10 mol kg -1时的不同离子水合模式不同程度地影响离子传输和弯曲度。在拉曼水带中观察到的氢键变化证实了这一点。最后,我们引入了弯曲度、孔内离子扩散率和电容之间的关系,以区分开路电压以及施加偏置电压下微孔和中孔内的电荷分布。 我们的研究结果表明,浓水电解质中的离子解离程度是决定碳电极电容器充电/放电速率性能的关键参数。这项研究有助于开发碳材料和兼容的电解质,以确保电容器满足所需的性能标准,同时可靠和高效。





































 京公网安备 11010802027423号
京公网安备 11010802027423号