Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FRA1 controls acinar cell plasticity during murine KrasG12D-induced pancreatic acinar to ductal metaplasia
Developmental Cell ( IF 10.7 ) Pub Date : 2024-08-22 , DOI: 10.1016/j.devcel.2024.07.021 Alina L Li 1 , Kensuke Sugiura 1 , Noriyuki Nishiwaki 1 , Kensuke Suzuki 2 , Dorsay Sadeghian 3 , Jun Zhao 3 , Anirban Maitra 3 , David Falvo 4 , Rohit Chandwani 4 , Jason R Pitarresi 5 , Peter A Sims 6 , Anil K Rustgi 1
Developmental Cell ( IF 10.7 ) Pub Date : 2024-08-22 , DOI: 10.1016/j.devcel.2024.07.021 Alina L Li 1 , Kensuke Sugiura 1 , Noriyuki Nishiwaki 1 , Kensuke Suzuki 2 , Dorsay Sadeghian 3 , Jun Zhao 3 , Anirban Maitra 3 , David Falvo 4 , Rohit Chandwani 4 , Jason R Pitarresi 5 , Peter A Sims 6 , Anil K Rustgi 1
Affiliation
|
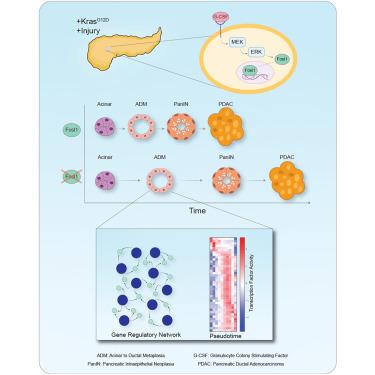
Acinar cells have been proposed as a cell-of-origin for pancreatic ductal adenocarcinoma (PDAC) after undergoing acinar-to-ductal metaplasia (ADM). ADM can be triggered by pancreatitis, causing acinar cells to de-differentiate to a ductal-like state. We identify FRA1 (gene name Fosl1) as the most active transcription factor during KrasG12D acute pancreatitis-mediated injury, and we have elucidated a functional role of FRA1 by generating an acinar-specific Fosl1 knockout mouse expressing KrasG12D. Using a gene regulatory network and pseudotime trajectory inferred from single-nuclei ATAC-seq and bulk RNA sequencing (RNA-seq), we hypothesized a regulatory model of the acinar-ADM-pancreatic intraepithelial neoplasia (PanIN) continuum and experimentally validated that Fosl1 knockout mice are delayed in the onset of ADM and neoplastic transformation. Our study also identifies that pro-inflammatory cytokines, such as granulocyte colony stimulating factor (G-CSF), can regulate FRA1 activity to modulate ADM. Our findings identify that FRA1 is a mediator of acinar cell plasticity and is critical for acinar cell de-differentiation and transformation.
中文翻译:

FRA1 控制小鼠 KrasG12D 诱导的胰腺腺泡到导管化生过程中腺泡细胞的可塑性
腺泡细胞已被提议作为腺泡-导管化生 (ADM) 后胰腺导管腺癌 (PDAC) 的起源细胞。ADM 可由胰腺炎触发,导致腺泡细胞去分化为导管样状态。我们确定 FRA1 (基因名称 Fosl1) 是 KrasG12D 急性胰腺炎介导的损伤期间最活跃的转录因子,并且我们通过产生表达 KrasG12D 的腺泡特异性 Fosl1 敲除小鼠阐明了 FRA1 的功能作用。使用从单核 ATAC-seq 和大量 RNA 测序 (RNA-seq) 推断的基因调控网络和伪时间轨迹,我们假设了腺泡-ADM-胰腺上皮内瘤变 (PanIN) 连续体的调节模型,并实验验证了 Fosl1 敲除小鼠在 ADM 和肿瘤转化的开始中延迟。我们的研究还发现,促炎细胞因子,如粒细胞集落刺激因子 (G-CSF),可以调节 FRA1 活性以调节 ADM。我们的研究结果确定 FRA1 是腺泡细胞可塑性的介质,对腺泡细胞去分化和转化至关重要。
更新日期:2024-08-22
中文翻译:

FRA1 控制小鼠 KrasG12D 诱导的胰腺腺泡到导管化生过程中腺泡细胞的可塑性
腺泡细胞已被提议作为腺泡-导管化生 (ADM) 后胰腺导管腺癌 (PDAC) 的起源细胞。ADM 可由胰腺炎触发,导致腺泡细胞去分化为导管样状态。我们确定 FRA1 (基因名称 Fosl1) 是 KrasG12D 急性胰腺炎介导的损伤期间最活跃的转录因子,并且我们通过产生表达 KrasG12D 的腺泡特异性 Fosl1 敲除小鼠阐明了 FRA1 的功能作用。使用从单核 ATAC-seq 和大量 RNA 测序 (RNA-seq) 推断的基因调控网络和伪时间轨迹,我们假设了腺泡-ADM-胰腺上皮内瘤变 (PanIN) 连续体的调节模型,并实验验证了 Fosl1 敲除小鼠在 ADM 和肿瘤转化的开始中延迟。我们的研究还发现,促炎细胞因子,如粒细胞集落刺激因子 (G-CSF),可以调节 FRA1 活性以调节 ADM。我们的研究结果确定 FRA1 是腺泡细胞可塑性的介质,对腺泡细胞去分化和转化至关重要。































 京公网安备 11010802027423号
京公网安备 11010802027423号