当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding non-covalent interactions in Ni-catalyzed reactions: Mechanistic insights into stereoselective tetrasubstituted allene synthesis
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-08-20 , DOI: 10.1016/j.checat.2024.101082 Seoyeon Kim , Da Seul Lee , Naeem Iqbal , Jaehan Bae , Ho Seong Hwang , Doohyun Baek , Sukwon Hong , Eun Jin Cho
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-08-20 , DOI: 10.1016/j.checat.2024.101082 Seoyeon Kim , Da Seul Lee , Naeem Iqbal , Jaehan Bae , Ho Seong Hwang , Doohyun Baek , Sukwon Hong , Eun Jin Cho
|
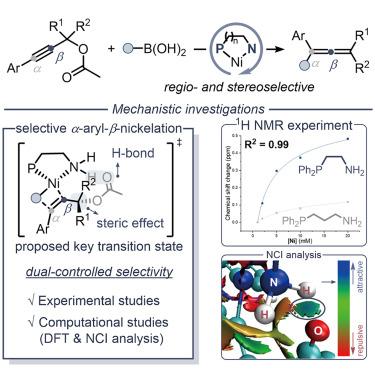
We report a nickel-catalyzed approach for the efficient synthesis of tetrasubstituted allenes from tertiary propargylic substrates, which remains a significant challenge due to competing substitution reactions and regioselectivity issues. Leveraging an optimized Ni-PˆN catalytic system, the notable non-covalent interactions (NCIs) overcome inherent stability issues and achieve regio- and stereoselectivity. Through a combination of experimental and computational analyses, we elucidate the stabilization mechanisms of the critical β -[Ni]-alkenyl intermediate from the reactions of arylated propargyl substrates. Our findings demonstrate that NCIs, in particular hydrogen bonding interactions between a unique free amino-type PˆN ligand and the substrate, are key to achieving precise control over the process. The efficiency of the process is greatly influenced by subtle differences in the ligand system through control of the bite angle and coordination length in the key nickel complex intermediates.
中文翻译:

了解镍催化反应中的非共价相互作用:立体选择性四取代丙二烯合成的机理见解
我们报告了一种镍催化方法,用于从叔炔丙底物中有效合成四取代丙二烯,由于竞争性取代反应和区域选择性问题,这仍然是一个重大挑战。利用优化的 Ni-PˆN 催化系统,显着的非共价相互作用 (NCI) 克服了固有的稳定性问题,并实现了区域选择性和立体选择性。通过实验和计算分析相结合,我们阐明了芳基化炔丙基底物反应中关键的 β-[Ni]-烯基中间体的稳定机制。我们的研究结果表明,NCI,特别是独特的游离氨基型 PˆN 配体与底物之间的氢键相互作用,是实现对该过程精确控制的关键。通过控制关键镍配合物中间体的咬合角和配位长度,配体系统的细微差异极大地影响了该过程的效率。
更新日期:2024-08-20
中文翻译:

了解镍催化反应中的非共价相互作用:立体选择性四取代丙二烯合成的机理见解
我们报告了一种镍催化方法,用于从叔炔丙底物中有效合成四取代丙二烯,由于竞争性取代反应和区域选择性问题,这仍然是一个重大挑战。利用优化的 Ni-PˆN 催化系统,显着的非共价相互作用 (NCI) 克服了固有的稳定性问题,并实现了区域选择性和立体选择性。通过实验和计算分析相结合,我们阐明了芳基化炔丙基底物反应中关键的 β-[Ni]-烯基中间体的稳定机制。我们的研究结果表明,NCI,特别是独特的游离氨基型 PˆN 配体与底物之间的氢键相互作用,是实现对该过程精确控制的关键。通过控制关键镍配合物中间体的咬合角和配位长度,配体系统的细微差异极大地影响了该过程的效率。































 京公网安备 11010802027423号
京公网安备 11010802027423号