Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-entropy electrolyte toward battery working under extreme conditions
Joule ( IF 38.6 ) Pub Date : 2024-08-20 , DOI: 10.1016/j.joule.2024.07.019 Meilong Wang , Mengting Zheng , Jun Lu , Ya You
Joule ( IF 38.6 ) Pub Date : 2024-08-20 , DOI: 10.1016/j.joule.2024.07.019 Meilong Wang , Mengting Zheng , Jun Lu , Ya You
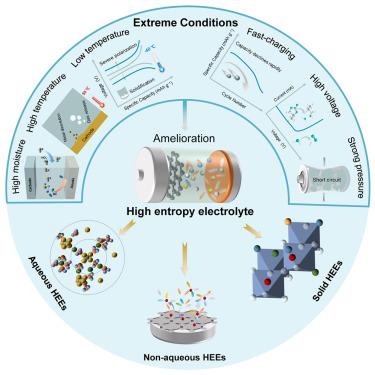
|
With the rapid expansion of battery applications, the demand for operating batteries in extreme conditions (e.g., high/low temperatures, high voltages, fast charging, etc.) is ever rising. The electrolyte is a key component in batteries, with properties that have far-reaching effects on the battery performance. Yet, according to general design principles of the electrolyte, operation under such harsh environments seems infeasible. In response, battery communities are scrambling to develop new concepts and theories. From the thermodynamics point of view, the free energy of the mixed system seriously affects the formation of the solvation structure of the liquid electrolyte, and the stability of the solid electrolyte is largely governed by entropy. Tuning the entropy of the electrolyte, in principle, represents a viable strategy to promote electrolyte features. Here, the entropy-tuning effect of electrolytes for batteries working under extreme conditions is thoroughly discussed in respect of aqueous, non-aqueous, and solid-state electrolytes. We believe that such a perspective will spark new thinking on the rational design of electrolytes aimed for use under extreme conditions.
中文翻译:

高熵电解质促进电池在极端条件下工作
随着电池应用的快速扩展,对电池在极端条件下(如高低温、高电压、快速充电等)运行的需求不断上升。电解质是电池的关键成分,其特性对电池的性能有着深远的影响。然而,根据电解质的一般设计原理,在如此恶劣的环境下运行似乎是不可行的。作为回应,电池界正在争先恐后地开发新的概念和理论。从热力学角度来看,混合体系的自由能严重影响液体电解质溶剂化结构的形成,而固体电解质的稳定性很大程度上受熵的控制。原则上,调节电解质的熵是促进电解质特性的可行策略。在这里,针对水性、非水性和固态电解质,对极端条件下工作的电池电解质的熵调节效应进行了深入讨论。我们相信,这样的观点将引发对旨在极端条件下使用的电解质的合理设计的新思考。
更新日期:2024-08-20
中文翻译:

高熵电解质促进电池在极端条件下工作
随着电池应用的快速扩展,对电池在极端条件下(如高低温、高电压、快速充电等)运行的需求不断上升。电解质是电池的关键成分,其特性对电池的性能有着深远的影响。然而,根据电解质的一般设计原理,在如此恶劣的环境下运行似乎是不可行的。作为回应,电池界正在争先恐后地开发新的概念和理论。从热力学角度来看,混合体系的自由能严重影响液体电解质溶剂化结构的形成,而固体电解质的稳定性很大程度上受熵的控制。原则上,调节电解质的熵是促进电解质特性的可行策略。在这里,针对水性、非水性和固态电解质,对极端条件下工作的电池电解质的熵调节效应进行了深入讨论。我们相信,这样的观点将引发对旨在极端条件下使用的电解质的合理设计的新思考。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号