Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identifying the lithium bond and lithium ionic bond in electrolytes
Chem ( IF 19.1 ) Pub Date : 2024-08-20 , DOI: 10.1016/j.chempr.2024.07.016 Nan Yao , Xiang Chen , Shu-Yu Sun , Yu-Chen Gao , Legeng Yu , Yan-Bin Gao , Wei-Lin Li , Qiang Zhang
Chem ( IF 19.1 ) Pub Date : 2024-08-20 , DOI: 10.1016/j.chempr.2024.07.016 Nan Yao , Xiang Chen , Shu-Yu Sun , Yu-Chen Gao , Legeng Yu , Yan-Bin Gao , Wei-Lin Li , Qiang Zhang
|
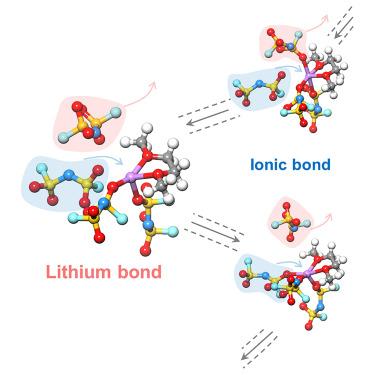
Lithium (Li) chemistry has been a significant branch of modern chemistry due to its wide and critical applications, such as Li batteries. Similar to the hydrogen (H) bond, the Li bond is the central topic of Li chemistry, but its nature is far from clear. Herein, the fundamental chemistry of the Li bond is systematically investigated, taking Li battery electrolytes as an example. Specifically, the Li bond and Li ionic bond can be differentiated according to nuclear magnetic resonance spectroscopy as 7 Li chemical shifts exhibit a downfield and upfield shift, respectively. The downfield shift indicates an electron localization effect of the Li bond beyond electrostatic interactions, which mainly dominate the ionic bond. Bond and electronic structure analyses further verify the difference between these two bonds. This work establishes principles to identify the Li bond and Li ionic bond, which contribute to Li chemistry and related applications, such as Li batteries.
中文翻译:

识别电解质中的锂键和锂离子键
锂 (Li) 化学因其广泛而关键的应用(例如锂电池)而成为现代化学的一个重要分支。与氢 (H) 键类似,Li 键是 Li 化学的中心主题,但其性质尚不清楚。本文以锂电池电解质为例,系统研究了锂键的基本化学性质。具体来说,Li键和Li离子键可以根据核磁共振波谱进行区分,因为7Li化学位移分别表现出下场和上场位移。下场偏移表明 Li 键的电子局域效应超越了静电相互作用,静电相互作用主要主导离子键。键和电子结构分析进一步验证了这两种键之间的差异。这项工作建立了识别锂键和锂离子键的原理,这有助于锂化学和相关应用,例如锂电池。
更新日期:2024-08-20
中文翻译:

识别电解质中的锂键和锂离子键
锂 (Li) 化学因其广泛而关键的应用(例如锂电池)而成为现代化学的一个重要分支。与氢 (H) 键类似,Li 键是 Li 化学的中心主题,但其性质尚不清楚。本文以锂电池电解质为例,系统研究了锂键的基本化学性质。具体来说,Li键和Li离子键可以根据核磁共振波谱进行区分,因为7Li化学位移分别表现出下场和上场位移。下场偏移表明 Li 键的电子局域效应超越了静电相互作用,静电相互作用主要主导离子键。键和电子结构分析进一步验证了这两种键之间的差异。这项工作建立了识别锂键和锂离子键的原理,这有助于锂化学和相关应用,例如锂电池。































 京公网安备 11010802027423号
京公网安备 11010802027423号