当前位置:
X-MOL 学术
›
Gastroenterology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fazirsiran for Adults With Alpha-1 Antitrypsin Deficiency Liver Disease: A Phase 2 Placebo Controlled Trial (SEQUOIA)
Gastroenterology ( IF 25.7 ) Pub Date : 2024-07-02 , DOI: 10.1053/j.gastro.2024.06.028 Virginia C Clark 1 , Charlie Strange 2 , Pavel Strnad 3 , Antonio J Sanchez 4 , Paul Kwo 5 , Vitor Magno Pereira 6 , Bart van Hoek 7 , Igor Barjaktarevic 8 , Angelo Guido Corsico 9 , Monica Pons 10 , Monica Goldklang 11 , Meagan Gray 12 , Brooks Kuhn 13 , Hugo E Vargas 14 , John M Vierling 15 , Raj Vuppalanchi 16 , Mark Brantly 17 , Naomi Kappe 18 , Ting Chang 19 , Thomas Schluep 19 , Rong Zhou 19 , James Hamilton 19 , Javier San Martin 19 , Rohit Loomba 20
中文翻译:

Fazirsiran 治疗 Alpha-1 抗胰蛋白酶缺乏症肝病成人患者:2 期安慰剂对照试验 (SEQUOIA)
纯合子 ZZ α-1 抗胰蛋白酶 (AAT) 缺陷在肝细胞中产生突变的 AAT (Z-AAT) 蛋白,导致进行性肝纤维化。我们评估了一种研究性 RNA 干扰治疗药物 fazirsiran 的安全性和有效性,该疗法可降解 Z-AAT 信使 RNA,减少有害蛋白质合成。
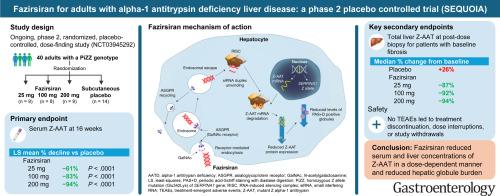
这项正在进行的 2 期研究将 40 名患者随机分配到皮下注射安慰剂或 fazirsiran 25、100 或 200 毫克。主要终点是血清 Z-AAT 浓度从基线到第 16 周的百分比变化。基线肝活检纤维化患者在第 1 天、第 4 周和每 12 周接受治疗,并在第 48 、 72 或 96 周或之后进行第二次肝活检。无纤维化的患者在第 1 天和第 4 周接受了 2 剂。
在第 16 周,与安慰剂相比,fazirsiran 25、100 和 200 mg 的血清 Z-AAT 浓度的最小二乘平均百分比下降分别为 -61% 、 -83% 和 -94% (均 P < .0001)。疗效持续到第 52 周。在给药后肝活检时,fazirsiran 将肝脏 Z-AAT 浓度中位数降低了 93%,而安慰剂组增加了 26%。所有 fazirsiran 治疗的患者肝球负荷的组织学水平均较基线降低。fazirsiran 组和安慰剂组 12 例患者中有 5 例和 8 例患者中有 0 例门静脉炎改善,基线评分分别为 >0。病毒性肝炎评分组织学数据的组织学荟萃分析显示,fazirsiran 组和安慰剂组 14 例中有 7 例和 8 例纤维化患者中有 3 例在基线时分别提高了 >F0 >1 分。无不良事件导致停药,肺功能检查保持稳定。
Fazirsiran 以剂量依赖性方式降低血清和肝脏 Z-AAT 浓度,并减轻肝球负荷。(ClinicalTrials.gov,第 NCT03945292 号)
更新日期:2024-07-02
Gastroenterology ( IF 25.7 ) Pub Date : 2024-07-02 , DOI: 10.1053/j.gastro.2024.06.028 Virginia C Clark 1 , Charlie Strange 2 , Pavel Strnad 3 , Antonio J Sanchez 4 , Paul Kwo 5 , Vitor Magno Pereira 6 , Bart van Hoek 7 , Igor Barjaktarevic 8 , Angelo Guido Corsico 9 , Monica Pons 10 , Monica Goldklang 11 , Meagan Gray 12 , Brooks Kuhn 13 , Hugo E Vargas 14 , John M Vierling 15 , Raj Vuppalanchi 16 , Mark Brantly 17 , Naomi Kappe 18 , Ting Chang 19 , Thomas Schluep 19 , Rong Zhou 19 , James Hamilton 19 , Javier San Martin 19 , Rohit Loomba 20
Affiliation
|
Background & Aims
Homozygous ZZ alpha-1 antitrypsin (AAT) deficiency produces mutant AAT (Z-AAT) proteins in hepatocytes, leading to progressive liver fibrosis. We evaluated the safety and efficacy of an investigational RNA interference therapeutic, fazirsiran, that degrades Z-AAT messenger RNA, reducing deleterious protein synthesis.Methods
This ongoing, phase 2 study randomized 40 patients to subcutaneous placebo or fazirsiran 25, 100, or 200 mg. The primary endpoint was percent change in serum Z-AAT concentration from baseline to week 16. Patients with fibrosis on baseline liver biopsy received treatment on day 1, at week 4, and then every 12 weeks and had a second liver biopsy at or after weeks 48, 72, or 96. Patients without fibrosis received 2 doses on day 1 and at week 4.Results
At week 16, least-squares mean percent declines in serum Z-AAT concentration were −61%, −83%, and −94% with fazirsiran 25, 100, and 200 mg, respectively, vs placebo (all P < .0001). Efficacy was sustained through week 52. At postdose liver biopsy, fazirsiran reduced median liver Z-AAT concentration by 93% compared with an increase of 26% with placebo. All fazirsiran-treated patients had histologic reduction from baseline in hepatic globule burden. Portal inflammation improved in 5 of 12 and 0 of 8 patients with a baseline score of >0 in the fazirsiran and placebo groups, respectively. Histologic meta-analysis of histologic data in viral hepatitis score improved by >1 point in 7 of 14 and 3 of 8 patients with fibrosis of >F0 at baseline in the fazirsiran and placebo groups, respectively. No adverse events led to discontinuation, and pulmonary function tests remained stable.Conclusions
Fazirsiran reduced serum and liver concentrations of Z-AAT in a dose-dependent manner and reduced hepatic globule burden. (ClinicalTrials.gov, Number NCT03945292)中文翻译:

Fazirsiran 治疗 Alpha-1 抗胰蛋白酶缺乏症肝病成人患者:2 期安慰剂对照试验 (SEQUOIA)
背景和目标
纯合子 ZZ α-1 抗胰蛋白酶 (AAT) 缺陷在肝细胞中产生突变的 AAT (Z-AAT) 蛋白,导致进行性肝纤维化。我们评估了一种研究性 RNA 干扰治疗药物 fazirsiran 的安全性和有效性,该疗法可降解 Z-AAT 信使 RNA,减少有害蛋白质合成。
方法
这项正在进行的 2 期研究将 40 名患者随机分配到皮下注射安慰剂或 fazirsiran 25、100 或 200 毫克。主要终点是血清 Z-AAT 浓度从基线到第 16 周的百分比变化。基线肝活检纤维化患者在第 1 天、第 4 周和每 12 周接受治疗,并在第 48 、 72 或 96 周或之后进行第二次肝活检。无纤维化的患者在第 1 天和第 4 周接受了 2 剂。
结果
在第 16 周,与安慰剂相比,fazirsiran 25、100 和 200 mg 的血清 Z-AAT 浓度的最小二乘平均百分比下降分别为 -61% 、 -83% 和 -94% (均 P < .0001)。疗效持续到第 52 周。在给药后肝活检时,fazirsiran 将肝脏 Z-AAT 浓度中位数降低了 93%,而安慰剂组增加了 26%。所有 fazirsiran 治疗的患者肝球负荷的组织学水平均较基线降低。fazirsiran 组和安慰剂组 12 例患者中有 5 例和 8 例患者中有 0 例门静脉炎改善,基线评分分别为 >0。病毒性肝炎评分组织学数据的组织学荟萃分析显示,fazirsiran 组和安慰剂组 14 例中有 7 例和 8 例纤维化患者中有 3 例在基线时分别提高了 >F0 >1 分。无不良事件导致停药,肺功能检查保持稳定。
结论
Fazirsiran 以剂量依赖性方式降低血清和肝脏 Z-AAT 浓度,并减轻肝球负荷。(ClinicalTrials.gov,第 NCT03945292 号)































 京公网安备 11010802027423号
京公网安备 11010802027423号