当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proteolysis-targeting drug delivery system (ProDDS): integrating targeted protein degradation concepts into formulation design
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2024-08-22 , DOI: 10.1039/d4cs00411f Yu Chen 1, 2, 3 , Fengyuan Liu 1 , Samira Pal 1 , Quanyin Hu 1, 2, 3
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2024-08-22 , DOI: 10.1039/d4cs00411f Yu Chen 1, 2, 3 , Fengyuan Liu 1 , Samira Pal 1 , Quanyin Hu 1, 2, 3
Affiliation
|
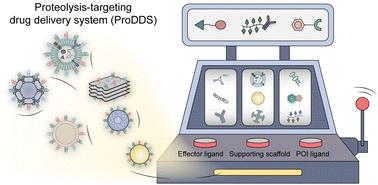
Targeted protein degradation (TPD) has emerged as a revolutionary paradigm in drug discovery and development, offering a promising avenue to tackle challenging therapeutic targets. Unlike traditional drug discovery approaches that focus on inhibiting protein function, TPD aims to eliminate proteins of interest (POIs) using modular chimeric structures. This is achieved through the utilization of proteolysis-targeting chimeras (PROTACs), which redirect POIs to E3 ubiquitin ligases, rendering them for degradation by the cellular ubiquitin–proteasome system (UPS). Additionally, other TPD technologies such as lysosome-targeting chimeras (LYTACs) and autophagy-based protein degraders facilitate the transportation of proteins to endo-lysosomal or autophagy-lysosomal pathways for degradation, respectively. Despite significant growth in preclinical TPD research, many chimeras fail to progress beyond this stage in the drug development. Various factors contribute to the limited success of TPD agents, including a significant hurdle of inadequate delivery to the target site. Integrating TPD into delivery platforms could surmount the challenges of in vivo applications of TPD strategies by reshaping their pharmacokinetics and pharmacodynamic profiles. These proteolysis-targeting drug delivery systems (ProDDSs) exhibit superior delivery performance, enhanced targetability, and reduced off-tissue side effects. In this review, we will survey the latest progress in TPD-inspired drug delivery systems, highlight the importance of introducing delivery ideas or technologies to the development of protein degraders, outline design principles of protein degrader-inspired delivery systems, discuss the current challenges, and provide an outlook on future opportunities in this field.
中文翻译:

蛋白水解靶向给药系统(ProDDS):将靶向蛋白降解概念融入制剂设计
靶向蛋白质降解(TPD)已成为药物发现和开发的革命性范例,为解决具有挑战性的治疗目标提供了一条有前途的途径。与专注于抑制蛋白质功能的传统药物发现方法不同,TPD 旨在使用模块化嵌合结构消除感兴趣的蛋白质 (POI)。这是通过利用蛋白水解靶向嵌合体 (PROTAC) 来实现的,它将 POI 重定向到 E3 泛素连接酶,使它们被细胞泛素-蛋白酶体系统 (UPS) 降解。此外,其他 TPD 技术,例如溶酶体靶向嵌合体 (LYTAC) 和基于自噬的蛋白质降解剂,分别促进蛋白质转运至内溶酶体或自噬-溶酶体途径进行降解。尽管临床前 TPD 研究显着增长,但许多嵌合体未能在药物开发的这一阶段取得进展。多种因素导致 TPD 药物的成功有限,其中包括无法充分递送至目标部位的重大障碍。将 TPD 整合到递送平台中可以通过重塑其药代动力学和药效学特征来克服 TPD 策略体内应用的挑战。这些蛋白水解靶向药物递送系统 (ProDDS) 表现出卓越的递送性能、增强的靶向性和减少的组织外副作用。在这篇综述中,我们将调查TPD启发的药物递送系统的最新进展,强调引入递送理念或技术对蛋白质降解剂开发的重要性,概述蛋白质降解剂启发的递送系统的设计原则,讨论当前的挑战,并对该领域的未来机会进行展望。
更新日期:2024-08-22
中文翻译:

蛋白水解靶向给药系统(ProDDS):将靶向蛋白降解概念融入制剂设计
靶向蛋白质降解(TPD)已成为药物发现和开发的革命性范例,为解决具有挑战性的治疗目标提供了一条有前途的途径。与专注于抑制蛋白质功能的传统药物发现方法不同,TPD 旨在使用模块化嵌合结构消除感兴趣的蛋白质 (POI)。这是通过利用蛋白水解靶向嵌合体 (PROTAC) 来实现的,它将 POI 重定向到 E3 泛素连接酶,使它们被细胞泛素-蛋白酶体系统 (UPS) 降解。此外,其他 TPD 技术,例如溶酶体靶向嵌合体 (LYTAC) 和基于自噬的蛋白质降解剂,分别促进蛋白质转运至内溶酶体或自噬-溶酶体途径进行降解。尽管临床前 TPD 研究显着增长,但许多嵌合体未能在药物开发的这一阶段取得进展。多种因素导致 TPD 药物的成功有限,其中包括无法充分递送至目标部位的重大障碍。将 TPD 整合到递送平台中可以通过重塑其药代动力学和药效学特征来克服 TPD 策略体内应用的挑战。这些蛋白水解靶向药物递送系统 (ProDDS) 表现出卓越的递送性能、增强的靶向性和减少的组织外副作用。在这篇综述中,我们将调查TPD启发的药物递送系统的最新进展,强调引入递送理念或技术对蛋白质降解剂开发的重要性,概述蛋白质降解剂启发的递送系统的设计原则,讨论当前的挑战,并对该领域的未来机会进行展望。































 京公网安备 11010802027423号
京公网安备 11010802027423号