当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition-metal-catalyzed enantioselective C–N cross-coupling
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2024-08-22 , DOI: 10.1039/d4cs00102h Jia Feng 1 , Long-Long Xi 1 , Chuan-Jun Lu 1 , Ren-Rong Liu 1, 2, 3
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2024-08-22 , DOI: 10.1039/d4cs00102h Jia Feng 1 , Long-Long Xi 1 , Chuan-Jun Lu 1 , Ren-Rong Liu 1, 2, 3
Affiliation
|
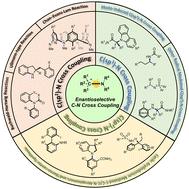
Chiral amine scaffolds are among the most important building blocks in natural products, drug molecules, and functional materials, which have prompted chemists to focus more on their synthesis. Among the accomplishments in chiral amine synthesis, transition-metal-catalyzed enantioselective C–N cross-coupling is considered one of the most efficient protocols. This approach combines traditional C(sp2)–N cross-coupling methods (such as the Buchwald–Hartwig reaction Ullmann-type reaction, and Chan–Evans–Lam reaction), aryliodonium salt chemistry and radical chemistry, providing an attractive pathway to a wide range of structurally diverse chiral amines with high enantioselectivity. This review summarizes the established protocols and offers a comprehensive outlook on the promising enantioselective C–N cross-coupling reaction.
中文翻译:

过渡金属催化的对映选择性 C-N 交叉偶联
手性胺支架是天然产物、药物分子和功能材料中最重要的构建模块之一,这促使化学家更加关注其合成。在手性胺合成的成就中,过渡金属催化的对映选择性 C-N 交叉偶联被认为是最有效的方案之一。该方法结合了传统的C(sp 2 )–N交叉偶联方法(例如Buchwald–Hartwig反应、乌尔曼型反应和Chan–Evans–Lam反应)、芳基碘鎓盐化学和自由基化学,提供了一条有吸引力的途径具有高对映选择性的各种结构多样的手性胺。这篇综述总结了已建立的方案,并对有前途的对映选择性 C-N 交叉偶联反应提供了全面的展望。
更新日期:2024-08-22
中文翻译:

过渡金属催化的对映选择性 C-N 交叉偶联
手性胺支架是天然产物、药物分子和功能材料中最重要的构建模块之一,这促使化学家更加关注其合成。在手性胺合成的成就中,过渡金属催化的对映选择性 C-N 交叉偶联被认为是最有效的方案之一。该方法结合了传统的C(sp 2 )–N交叉偶联方法(例如Buchwald–Hartwig反应、乌尔曼型反应和Chan–Evans–Lam反应)、芳基碘鎓盐化学和自由基化学,提供了一条有吸引力的途径具有高对映选择性的各种结构多样的手性胺。这篇综述总结了已建立的方案,并对有前途的对映选择性 C-N 交叉偶联反应提供了全面的展望。































 京公网安备 11010802027423号
京公网安备 11010802027423号