当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H-assisted CO2 dissociation on PdnPt(4−n)/In2O3 catalysts: a density functional theory study
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-22 , DOI: 10.1039/d4cp02389g Xiaowen Wang 1 , Jiaying Pan 1 , Haiqiao Wei 1, 2 , Wenjia Li 3 , Jun Zhao 2, 4 , Zhen Hu 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-08-22 , DOI: 10.1039/d4cp02389g Xiaowen Wang 1 , Jiaying Pan 1 , Haiqiao Wei 1, 2 , Wenjia Li 3 , Jun Zhao 2, 4 , Zhen Hu 1
Affiliation
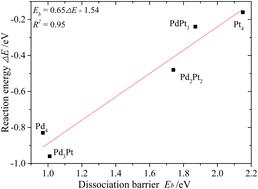
|
CO2 hydrogenation into valuable chemical compounds can effectively address the issues of greenhouse gas emissions and energy scarcity. The activation and dissociation processes of CO2 are crucial for its reduction reactions, but the effects of *H adatoms on the C–O cleavage are still confusing. This study investigates the H-assisted CO2 dissociation pathways on the PdnPt(4−n)/In2O3 (n = 0–4) catalysts via DFT calculation. Initially, the adsorption properties of *H2, *COOH, and *HCOO species are calculated. Then, two H-assisted CO2 dissociation channels, i.e., *CO2 + *H → *COOH → *CO + *OH and *CO2 + *H → *HCOO → *CHO + *O, are studied. Results show that Pt and Pd promote the CO2 hydrogenation and C–O bond cleavage reactions, respectively. In comparison to CO2 direct dissociation, the COOH-mediated and HCOO-mediated channels facilitate and impede the C–O bond cleavage, respectively. Overall, the Pd3Pt/In2O3 constituent is suggested for the H-assisted CO2 dissociation reaction. The electronic effects of the PdnPt(4−n) bimetals adjust the stabilities of the intermediates and barriers of the elementary steps, and the interactions between PdnPt(4−n) and In2O3 provide extra sites for the adsorbates and reaction steps. This study reveals the effects of *H on the C–O bond dissociation processes and provides useful insight into designing PdPt/In2O3 catalysts for CO2 hydrogenation reactions.
中文翻译:

PdnPt(4−n)/In2O3 催化剂上 H 辅助 CO2 解离:密度泛函理论研究
CO 2加氢成有价值的化合物可以有效解决温室气体排放和能源短缺问题。 CO 2的活化和解离过程对其还原反应至关重要,但*H吸附原子对C-O裂解的影响仍然令人困惑。本研究通过DFT 计算研究了Pd n Pt (4− n ) /In 2 O 3 ( n = 0–4) 催化剂上H 辅助的CO 2解离途径。首先,计算*H 2 、*COOH和*HCOO物质的吸附性质。然后,研究了两种H辅助的CO 2解离通道,即*CO 2 + *H→*COOH→*CO + *OH和*CO 2 + *H→*HCOO→*CHO + *O。结果表明Pt和Pd分别促进CO 2加氢和C-O键断裂反应。与CO 2直接解离相比,COOH介导和HCOO介导的通道分别促进和阻碍C-O键裂解。总体而言,建议将Pd 3 Pt/In 2 O 3成分用于H辅助CO 2解离反应。 Pd n Pt (4− n )双金属的电子效应调节基本步骤的中间体和势垒的稳定性,Pd n Pt (4− n )和 In 2 O 3之间的相互作用为吸附物提供了额外的位点和反应步骤。这项研究揭示了 *H 对 C-O 键解离过程的影响,并为设计用于 CO 2加氢反应的 PdPt/In 2 O 3催化剂提供了有用的见解。
更新日期:2024-08-27
中文翻译:

PdnPt(4−n)/In2O3 催化剂上 H 辅助 CO2 解离:密度泛函理论研究
CO 2加氢成有价值的化合物可以有效解决温室气体排放和能源短缺问题。 CO 2的活化和解离过程对其还原反应至关重要,但*H吸附原子对C-O裂解的影响仍然令人困惑。本研究通过DFT 计算研究了Pd n Pt (4− n ) /In 2 O 3 ( n = 0–4) 催化剂上H 辅助的CO 2解离途径。首先,计算*H 2 、*COOH和*HCOO物质的吸附性质。然后,研究了两种H辅助的CO 2解离通道,即*CO 2 + *H→*COOH→*CO + *OH和*CO 2 + *H→*HCOO→*CHO + *O。结果表明Pt和Pd分别促进CO 2加氢和C-O键断裂反应。与CO 2直接解离相比,COOH介导和HCOO介导的通道分别促进和阻碍C-O键裂解。总体而言,建议将Pd 3 Pt/In 2 O 3成分用于H辅助CO 2解离反应。 Pd n Pt (4− n )双金属的电子效应调节基本步骤的中间体和势垒的稳定性,Pd n Pt (4− n )和 In 2 O 3之间的相互作用为吸附物提供了额外的位点和反应步骤。这项研究揭示了 *H 对 C-O 键解离过程的影响,并为设计用于 CO 2加氢反应的 PdPt/In 2 O 3催化剂提供了有用的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号