当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to 4-((Pyridin-2-yl)amino)quinazolinones via Annulation of 2-Aminobenzonitriles with N′-(Pyridin-2-yl)-N,N-dimethyl Ureas
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-08-21 , DOI: 10.1021/acs.joc.4c00974 Svetlana O Baykova 1 , Sergey V Baykov 1 , Olga V Solodyankina 1 , Vadim P Boyarskiy 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-08-21 , DOI: 10.1021/acs.joc.4c00974 Svetlana O Baykova 1 , Sergey V Baykov 1 , Olga V Solodyankina 1 , Vadim P Boyarskiy 1
Affiliation
|
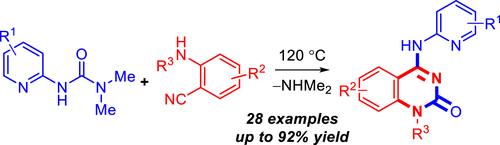
We have developed a convenient protocol for synthesizing N-(2-pyridyl)-substituted 4-(amino)quinazolin-2(1H)-ones by reacting N,N-dimethyl-N′-pyridylureas with 2-aminobenzonitriles. The method relies on the ability of N,N-dimethyl-N′-pyridyl/quinolinyl ureas to act as masked isocyanates under thermal activation, followed by a Dimroth rearrangement of 4-imino-3-(hetaryl)-3,4-dihydroquinazolin-2(1H)-ones. Conducted at 120 °C, either in DMF or under solvent-free conditions, this approach has produced 28 derivatives of 4-aminoquinazolinones, featuring pyridine or quinoline substituents, with yields of up to 92%.
中文翻译:

通过 2-氨基苯甲腈与 N'-(吡啶-2-基)-N,N-二甲基脲成环获得 4-((吡啶-2-基)氨基)喹唑啉酮
我们开发了一种通过N , N-二甲基-N'-吡啶脲与 2-氨基苯甲腈反应合成N- (2-吡啶基)-取代的 4-(氨基)喹唑啉-2(1 H )-酮的简便方案。该方法依赖于N , N-二甲基-N'-吡啶基/喹啉基脲在热活化下充当掩蔽异氰酸酯的能力,然后进行 4-亚氨基-3-(杂芳基)-3,4-二氢喹唑啉的 Dimroth 重排-2(1 H )-1。该方法在 120 °C、DMF 或无溶剂条件下进行,生成了 28 种带有吡啶或喹啉取代基的 4-氨基喹唑啉酮衍生物,产率高达 92%。
更新日期:2024-08-21
中文翻译:

通过 2-氨基苯甲腈与 N'-(吡啶-2-基)-N,N-二甲基脲成环获得 4-((吡啶-2-基)氨基)喹唑啉酮
我们开发了一种通过N , N-二甲基-N'-吡啶脲与 2-氨基苯甲腈反应合成N- (2-吡啶基)-取代的 4-(氨基)喹唑啉-2(1 H )-酮的简便方案。该方法依赖于N , N-二甲基-N'-吡啶基/喹啉基脲在热活化下充当掩蔽异氰酸酯的能力,然后进行 4-亚氨基-3-(杂芳基)-3,4-二氢喹唑啉的 Dimroth 重排-2(1 H )-1。该方法在 120 °C、DMF 或无溶剂条件下进行,生成了 28 种带有吡啶或喹啉取代基的 4-氨基喹唑啉酮衍生物,产率高达 92%。































 京公网安备 11010802027423号
京公网安备 11010802027423号