当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiazolyl-Substituted Isomeric Benzodithiophenes Boost the Photovoltaic Performance of Polymer Donors
Macromolecules ( IF 5.1 ) Pub Date : 2024-08-20 , DOI: 10.1021/acs.macromol.4c01474 Kangqiao Ma 1 , Huazhe Liang 1 , Yuxin Wang 1 , Xinyuan Jia 1 , Wendi Shi 1 , Zhaoyang Yao 1 , Xiangjian Wan 1 , Chenxi Li 1 , Yongsheng Chen 1
Macromolecules ( IF 5.1 ) Pub Date : 2024-08-20 , DOI: 10.1021/acs.macromol.4c01474 Kangqiao Ma 1 , Huazhe Liang 1 , Yuxin Wang 1 , Xinyuan Jia 1 , Wendi Shi 1 , Zhaoyang Yao 1 , Xiangjian Wan 1 , Chenxi Li 1 , Yongsheng Chen 1
Affiliation
|
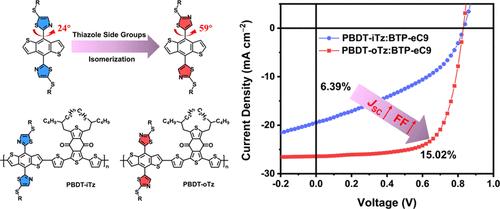
The two-dimensional side groups on benzodithiophene, especially thiophene derivatives, play a critical role in tailoring the bandgap, interchain packing, and photovoltaic outcomes of the most successful polymer donors. In light of the highly similar five-membered ring but vastly different electron-deficient properties, thiazole is expected to not only inherit the ability of the thiophene side group in conformation control but also be beneficial for constructing wide-bandgap polymer donors with deeper highest occupied molecular orbitals (HOMOs). Herein, two isomeric polymers (PBDT-oTz and PBDT-iTz) with different thiazolyl orientations on benzodithiophene are explored. A systematic investigation reveals that PBDT-oTz, featuring thiazolyl nitrogen far from benzodithiophene, achieves a deeper HOMO and appropriate molecular aggregation compared with its PBDT-iTz counterpart. Consequently, the PBDT-oTz-based device affords an excellent power conversion efficiency of 15.02%, much better than the 6.39% for PBDT-iTz. These results manifest the great effectiveness of thiazole in constructing high-performance wide-bandgap polymer donors.
中文翻译:

噻唑基取代的异构苯并二噻吩可提高聚合物供体的光伏性能
苯并二噻吩(尤其是噻吩衍生物)上的二维侧基在调整最成功的聚合物供体的带隙、链间堆积和光伏结果方面发挥着关键作用。鉴于五元环高度相似但缺电子性质截然不同,噻唑有望不仅继承噻吩侧基的构象控制能力,而且有利于构建具有更深最高占据的宽带隙聚合物供体分子轨道(HOMO)。在此,探索了苯并二噻吩上具有不同噻唑基取向的两种异构聚合物(PBDT-oTz 和 PBDT-iTz)。系统研究表明,PBDT-oTz 具有远离苯并二噻吩的噻唑基氮,与 PBDT-iTz 对应物相比,PBDT-oTz 实现了更深的 HOMO 和适当的分子聚集。因此,基于 PBDT-oTz 的器件具有 15.02% 的出色功率转换效率,远优于 PBDT-iTz 的 6.39%。这些结果证明了噻唑在构建高性能宽带隙聚合物供体方面的巨大有效性。
更新日期:2024-08-20
中文翻译:

噻唑基取代的异构苯并二噻吩可提高聚合物供体的光伏性能
苯并二噻吩(尤其是噻吩衍生物)上的二维侧基在调整最成功的聚合物供体的带隙、链间堆积和光伏结果方面发挥着关键作用。鉴于五元环高度相似但缺电子性质截然不同,噻唑有望不仅继承噻吩侧基的构象控制能力,而且有利于构建具有更深最高占据的宽带隙聚合物供体分子轨道(HOMO)。在此,探索了苯并二噻吩上具有不同噻唑基取向的两种异构聚合物(PBDT-oTz 和 PBDT-iTz)。系统研究表明,PBDT-oTz 具有远离苯并二噻吩的噻唑基氮,与 PBDT-iTz 对应物相比,PBDT-oTz 实现了更深的 HOMO 和适当的分子聚集。因此,基于 PBDT-oTz 的器件具有 15.02% 的出色功率转换效率,远优于 PBDT-iTz 的 6.39%。这些结果证明了噻唑在构建高性能宽带隙聚合物供体方面的巨大有效性。































 京公网安备 11010802027423号
京公网安备 11010802027423号