当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrogen spillover through hydride transfer: the reaction of ZnO and ZrO2 with strong hydride donors
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2024-08-19 , DOI: 10.1039/d4cy00504j Michael Benz 1 , Osman Bunjaku 1 , Michal Nowakowski 2 , Alexander Allgaier 1 , Indro Biswas 3 , Joris van Slageren 1 , Matthias Bauer 2 , Deven P. Estes 1
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2024-08-19 , DOI: 10.1039/d4cy00504j Michael Benz 1 , Osman Bunjaku 1 , Michal Nowakowski 2 , Alexander Allgaier 1 , Indro Biswas 3 , Joris van Slageren 1 , Matthias Bauer 2 , Deven P. Estes 1
Affiliation
|
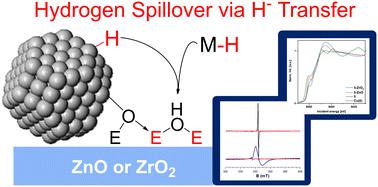
Hydrogen spillover, transfer of H2 from a metal surface to a support (often metal oxides), is pivotal for many heterogeneous catalytic processes, including Cu/ZnO and Cu/ZrO2 catalyzed methanol synthesis. Little is known about hydrogen spillover on ZnO or ZrO2, due to the high complexity of the metal–metal oxide interface. Here, we model hydrogen spillover on ZnO and ZrO2 by reacting them with molecular metal hydrides to see how the properties of the hydrides affect hydrogen spillover. While the good H· donors HV(CO)4dppe (1) and CpCr(CO)3H (2) do not react with the metal oxide surfaces, the strong hydride donors iBu2AlH (3), Cp2ZrHCl (4), and [HCu(PPh3)]6 (5) do reduce ZnO and ZrO2 to give defect sites with the same EPR signatures as obtained via hydrogen spillover. We also observe new M–O bonds to the surface using X-ray absorption spectroscopy (XAS). We propose that these metal oxides undergo hydrogen spillover via initial hydride transfer followed by tautomerization of the surface hydride, giving reduced sites and OH bonds. This mechanism is in contrast to the traditional spillover mechanism involving discrete proton- and electron transfer steps. We also observe that ZnO is easier to reduce than ZrO2, explaining the difficulty observing spillover on Cu/ZrO2.
中文翻译:

通过氢化物转移发生氢气溢出:ZnO 和 ZrO2 与强氢化物供体的反应
氢溢出,即H 2从金属表面转移到载体(通常是金属氧化物),对于许多多相催化过程(包括Cu/ZnO 和Cu/ZrO 2催化甲醇合成)至关重要。由于金属-金属氧化物界面的高度复杂性,人们对 ZnO 或 ZrO 2上的氢溢出知之甚少。在这里,我们通过将 ZnO 和 ZrO 2与分子金属氢化物反应来模拟氢溢出,以了解氢化物的性质如何影响氢溢出。良好的 H· 供体 HV(CO) 4 dppe ( 1 ) 和 CpCr(CO) 3 H ( 2 ) 不会与金属氧化物表面发生反应,而强氢化物供体i Bu 2 AlH ( 3 ) 、 Cp 2 ZrHCl ( 4 ) 和 [HCu(PPh 3 )] 6 ( 5 ) 确实还原了 ZnO 和 ZrO 2以给出具有与通过氢溢出获得的相同 EPR 特征的缺陷位点。我们还使用 X 射线吸收光谱 (XAS) 观察到表面新的 M-O 键。我们提出这些金属氧化物通过初始氢化物转移发生氢溢出,随后表面氢化物互变异构,产生还原位点和 OH 键。该机制与涉及离散质子和电子转移步骤的传统溢出机制形成对比。 我们还观察到 ZnO 比 ZrO 2更容易还原,这解释了观察 Cu/ZrO 2上溢出的困难。
更新日期:2024-08-19
中文翻译:

通过氢化物转移发生氢气溢出:ZnO 和 ZrO2 与强氢化物供体的反应
氢溢出,即H 2从金属表面转移到载体(通常是金属氧化物),对于许多多相催化过程(包括Cu/ZnO 和Cu/ZrO 2催化甲醇合成)至关重要。由于金属-金属氧化物界面的高度复杂性,人们对 ZnO 或 ZrO 2上的氢溢出知之甚少。在这里,我们通过将 ZnO 和 ZrO 2与分子金属氢化物反应来模拟氢溢出,以了解氢化物的性质如何影响氢溢出。良好的 H· 供体 HV(CO) 4 dppe ( 1 ) 和 CpCr(CO) 3 H ( 2 ) 不会与金属氧化物表面发生反应,而强氢化物供体i Bu 2 AlH ( 3 ) 、 Cp 2 ZrHCl ( 4 ) 和 [HCu(PPh 3 )] 6 ( 5 ) 确实还原了 ZnO 和 ZrO 2以给出具有与通过氢溢出获得的相同 EPR 特征的缺陷位点。我们还使用 X 射线吸收光谱 (XAS) 观察到表面新的 M-O 键。我们提出这些金属氧化物通过初始氢化物转移发生氢溢出,随后表面氢化物互变异构,产生还原位点和 OH 键。该机制与涉及离散质子和电子转移步骤的传统溢出机制形成对比。 我们还观察到 ZnO 比 ZrO 2更容易还原,这解释了观察 Cu/ZrO 2上溢出的困难。





































 京公网安备 11010802027423号
京公网安备 11010802027423号