当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A novel high-performance all-liquid formic acid redox fuel cell: simultaneously generating electricity and restoring the capacity of flow batteries
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-08-19 , DOI: 10.1039/d4ee02450h Dongbo Wei 1 , Lyuming Pan 1 , Jing Sun 2 , Meisheng Han 1 , Manrong Song 1 , Jincong Guo 1 , Qing Zhang 1 , Cailin Xiao 1 , Zheng Li 1 , Shuaibin Wan 3 , Yubai Li 4 , Lin Zeng 1 , Lei Wei 1 , Tianshou Zhao 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-08-19 , DOI: 10.1039/d4ee02450h Dongbo Wei 1 , Lyuming Pan 1 , Jing Sun 2 , Meisheng Han 1 , Manrong Song 1 , Jincong Guo 1 , Qing Zhang 1 , Cailin Xiao 1 , Zheng Li 1 , Shuaibin Wan 3 , Yubai Li 4 , Lin Zeng 1 , Lei Wei 1 , Tianshou Zhao 1
Affiliation
|
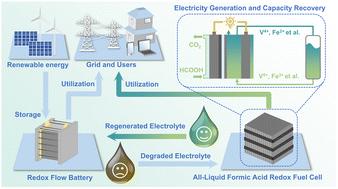
Conventional formic acid fuel cells rely on the oxygen reduction reaction (ORR) to generate the cathode potential. However, this approach is plagued by mixed-potential issues caused by formic acid crossover and poor cathodic electrochemical kinetics. To address these limitations, we propose an innovative design that replaces the oxygen with a liquid redox couple. By implementing the Bi-modified Pt/C electrocatalyst that can facilitate the formic acid oxidation reaction with robust CO tolerance, this novel redox fuel cell achieves an open circuit voltage and a peak power density of 1.23 V and 281.5 mW cm−2, respectively, representing a 55.7% and 235.1% improvement over the cell with the ORR cathode. Such performance metrics are among the highest recorded for formic acid fuel cells. Density functional theory calculations and a mathematical model are utilized to describe the increased activity of the produced catalysts and the cell working principles. Moreover, the redox fuel cell can be used to restore the capacity of flow batteries by using the degraded electrolyte as a cathode fuel. For example, the capacity of vanadium redox flow batteries can be recovered to 97.6% of the initial highest level after 400 cycle tests. Exhibiting high safety and convenience, this innovative cell offers a feasible, economically viable avenue for significantly extending the flow batteries’ cycle life.
中文翻译:

新型高性能全液体甲酸氧化还原燃料电池:同时发电并恢复液流电池容量
传统的甲酸燃料电池依靠氧还原反应(ORR)产生阴极电势。然而,这种方法受到甲酸交叉和较差的阴极电化学动力学引起的混合电位问题的困扰。为了解决这些限制,我们提出了一种创新设计,用液体氧化还原对代替氧气。通过使用能够促进甲酸氧化反应并具有强大的CO耐受性的Bi改性Pt/C电催化剂,这种新型氧化还原燃料电池的开路电压和峰值功率密度分别为1.23 V和281.5 mW cm -2 ,与采用 ORR 阴极的电池相比,性能分别提高了 55.7% 和 235.1%。这些性能指标是甲酸燃料电池的最高记录之一。利用密度泛函理论计算和数学模型来描述所生产的催化剂的活性增加和电池工作原理。此外,氧化还原燃料电池可通过使用降解的电解质作为阴极燃料来恢复液流电池的容量。例如,全钒氧化还原液流电池经过400次循环测试后容量可恢复至初始最高水平的97.6%。这种创新电池具有高安全性和便利性,为显着延长液流电池的循环寿命提供了可行且经济可行的途径。
更新日期:2024-08-19
中文翻译:

新型高性能全液体甲酸氧化还原燃料电池:同时发电并恢复液流电池容量
传统的甲酸燃料电池依靠氧还原反应(ORR)产生阴极电势。然而,这种方法受到甲酸交叉和较差的阴极电化学动力学引起的混合电位问题的困扰。为了解决这些限制,我们提出了一种创新设计,用液体氧化还原对代替氧气。通过使用能够促进甲酸氧化反应并具有强大的CO耐受性的Bi改性Pt/C电催化剂,这种新型氧化还原燃料电池的开路电压和峰值功率密度分别为1.23 V和281.5 mW cm -2 ,与采用 ORR 阴极的电池相比,性能分别提高了 55.7% 和 235.1%。这些性能指标是甲酸燃料电池的最高记录之一。利用密度泛函理论计算和数学模型来描述所生产的催化剂的活性增加和电池工作原理。此外,氧化还原燃料电池可通过使用降解的电解质作为阴极燃料来恢复液流电池的容量。例如,全钒氧化还原液流电池经过400次循环测试后容量可恢复至初始最高水平的97.6%。这种创新电池具有高安全性和便利性,为显着延长液流电池的循环寿命提供了可行且经济可行的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号