European Journal of Nuclear Medicine and Molecular Imaging ( IF 8.6 ) Pub Date : 2024-08-19 , DOI: 10.1007/s00259-024-06886-5 Yuan Yao 1 , Yanan Ren 2 , Xingguo Hou 1 , Pei Wang 3 , Jinyu Zhu 1 , Song Liu 1 , Xiaokun Ma 1 , Teli Liu 1 , Zhi Yang 1 , Hua Zhu 1 , Nan Li 1
|
Purpose
NB12 is a bispecific antibody that consists of two anti-programmed cell death-ligand 1 (PD-L1) nanobodies and two anti-programmed cell death-ligand 2 (PD-L2) nanobodies. The aim of this study was to design a novel tracer, [124I]I-NB12, targeting PD-L1/2 and perform preclinical evaluations to dynamically monitor PD-L1/2 expression for determining cancer patient responsiveness to ICI therapy.
Methods
NB12 was labelled with the radionuclide 124I at room temperature (RT). An in vitro binding assay was performed to assess the affinity of [124I]I-NB12 for PD-L1 and PD-L2. Cellular uptake, pharmacokinetic, and biodistribution experiments were performed to evaluate the biological properties. Micro-PET/CT imaging with [124I]I-NB12 was conducted at different time points. Immunohistochemical and haematoxylin and eosin (HE) staining experiments were carried out using tumour tissues. Routine blood, biochemical indices and major organ pathology were used to evaluate the biosafety of the tracers.
Results
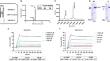
The radiochemical yield of [124I]I-NB12 was 84.62 ± 3.90%, and the radiochemical purity (RCP) was greater than 99%. [124I]I-NB12 had a high affinity for the PD-L1 (Kd = 19.82 nM) and PD-L2 (Kd = 2.93 nM). Cellular uptake experiments confirmed that the uptake of [124I]I-NB12 by A549-PDL1/2 cells was greater than that by A549 cells. The half-lives of the distribution phase and elimination phase were 0.26 h and 4.08 h, respectively. Micro-PET/CT showed significant [124I]I-NB12 uptake in the tumour region of A549-PDL1/2 tumour-bearing mice compared with A549 tumour-bearing mice 24 h postinjection. Immunohistochemical and HE staining experiments confirmed that tumour-bearing mice was successfully constructed.
Conclusion
We constructed a bispecific antibody that targets PD-L1 and PD-L2, namely, [124I]I-NB12. Biological evaluation revealed its specificity and affinity for PD-L1/2, and micro-PET/CT confirmed the feasibility of visualizing tumour PD-L1/2 in vivo. Using [124I]I-NB12 may be a promising strategy for identifying cancer patients that can potentially benefit from ICI therapy.
中文翻译:

124I标记的PD-L1和PD-L2双特异性抗体的构建和临床前评价
目的
NB12 是一种双特异性抗体,由两个抗程序性细胞死亡配体 1 (PD-L1) 纳米抗体和两个抗程序性细胞死亡配体 2 (PD-L2) 纳米抗体组成。本研究的目的是设计一种新型示踪剂 [ 124 I]I-NB12,靶向 PD-L1/2 并进行临床前评估以动态监测 PD-L1/2 表达,以确定癌症患者对 ICI 治疗的反应。
方法
NB12 在室温 (RT) 下用放射性核素124 I 标记。进行体外结合测定以评估 [ 124 I]I-NB12 对 PD-L1 和 PD-L2 的亲和力。进行细胞摄取、药代动力学和生物分布实验以评估生物学特性。在不同时间点进行[ 124 I]I-NB12 的微型 PET/CT 成像。使用肿瘤组织进行免疫组织化学和苏木精和伊红(HE)染色实验。采用血常规、生化指标和主要器官病理学指标评价示踪剂的生物安全性。
结果
[ 124 I]I-NB12的放射化学收率为84.62±3.90%,放射化学纯度(RCP)大于99%。 [ 124 I]I-NB12 对 PD-L1 (Kd = 19.82 nM) 和 PD-L2 (Kd = 2.93 nM) 具有高亲和力。细胞摄取实验证实A549-PDL1/2细胞对[ 124 I]I-NB12的摄取大于A549细胞。分布期和消除期的半衰期分别为0.26小时和4.08小时。 Micro-PET/CT显示注射后24小时,与A549荷瘤小鼠相比,A549-PDL1/2荷瘤小鼠的肿瘤区域有显着的[ 124 I]I-NB12摄取。免疫组化和HE染色实验证实荷瘤小鼠构建成功。
结论
我们构建了一种靶向 PD-L1 和 PD-L2 的双特异性抗体,即 [ 124 I]I-NB12。生物学评价揭示了其对PD-L1/2的特异性和亲和力,显微PET/CT证实了体内肿瘤PD-L1/2可视化的可行性。使用 [ 124 I]I-NB12 可能是识别可能受益于 ICI 治疗的癌症患者的一种有前途的策略。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号