当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Debate over Hard Carbon and Alloy Anodes Continues for Solid-State Sodium Batteries
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-08-19 , DOI: 10.1021/acsenergylett.4c01686 Xinyu Yu 1, 2 , Shiwei Chen 1, 2 , Bin Tang 3, 4 , Xun-Lu Li 1 , Jingying Zhou 1, 2 , Yushan Ren 1 , Jie Wei 1, 2 , Chengyin Yang 1, 2 , Yunlong Guo 1, 2 , Zhen Zhou 3 , Shou-Hang Bo 1, 5
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-08-19 , DOI: 10.1021/acsenergylett.4c01686 Xinyu Yu 1, 2 , Shiwei Chen 1, 2 , Bin Tang 3, 4 , Xun-Lu Li 1 , Jingying Zhou 1, 2 , Yushan Ren 1 , Jie Wei 1, 2 , Chengyin Yang 1, 2 , Yunlong Guo 1, 2 , Zhen Zhou 3 , Shou-Hang Bo 1, 5
Affiliation
|
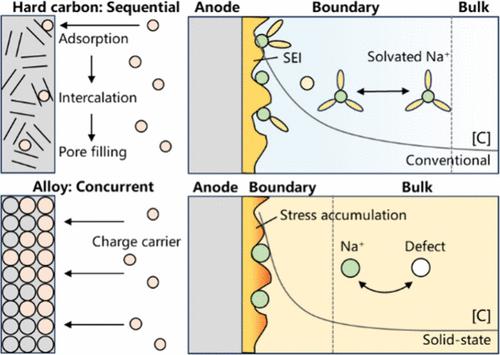
Although hard carbon anodes are known to outperform alloys in conventional sodium-ion batteries, this trend is reversed in solid-state sodium batteries due to the different underlying sodiation processes. Whereas the sodiation of hard carbon is triggered by Na cation (Na+) adsorption onto electrochemically active sites, that of alloys is driven by solid-state diffusion of Na+ and successive Na–alloy phase transformations. Thus, sodiation processes critically depend upon the chemical nature of Na+, which is solvated in liquids but is bonded at lattice sites in solid electrolytes. In addition, elucidating charge-transport and charge-transfer processes as well as electromechanical coupling at solid/solid interfaces (for solid-state batteries) remains an unresolved challenge. The transition in knowledge from well-investigated solid/liquid interfaces to solid-state sodium batteries is not straightforward. The exploration of hard carbon, alloys, and their composites requires further advancement. This perspective aids in streamlining the research efforts of battery communities, thereby accelerating the development of solid-state sodium batteries.
中文翻译:

关于固态钠电池硬碳和合金阳极的争论仍在继续
尽管众所周知,硬碳阳极在传统钠离子电池中的性能优于合金,但由于潜在的钠化过程不同,这种趋势在固态钠电池中却相反。硬碳的钠化是由钠阳离子 (Na + ) 吸附到电化学活性位点引发的,而合金的钠化是由 Na +的固态扩散和连续的钠合金相变驱动的。因此,钠化过程关键取决于Na +的化学性质,Na + 在液体中溶剂化,但在固体电解质的晶格位置键合。此外,阐明电荷传输和电荷转移过程以及固体/固体界面(对于固态电池)的机电耦合仍然是一个尚未解决的挑战。从深入研究的固/液界面到固态钠电池的知识转变并不简单。硬碳、合金及其复合材料的探索需要进一步推进。这一观点有助于简化电池界的研究工作,从而加速固态钠电池的发展。
更新日期:2024-08-19
中文翻译:

关于固态钠电池硬碳和合金阳极的争论仍在继续
尽管众所周知,硬碳阳极在传统钠离子电池中的性能优于合金,但由于潜在的钠化过程不同,这种趋势在固态钠电池中却相反。硬碳的钠化是由钠阳离子 (Na + ) 吸附到电化学活性位点引发的,而合金的钠化是由 Na +的固态扩散和连续的钠合金相变驱动的。因此,钠化过程关键取决于Na +的化学性质,Na + 在液体中溶剂化,但在固体电解质的晶格位置键合。此外,阐明电荷传输和电荷转移过程以及固体/固体界面(对于固态电池)的机电耦合仍然是一个尚未解决的挑战。从深入研究的固/液界面到固态钠电池的知识转变并不简单。硬碳、合金及其复合材料的探索需要进一步推进。这一观点有助于简化电池界的研究工作,从而加速固态钠电池的发展。































 京公网安备 11010802027423号
京公网安备 11010802027423号