当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of a Novel Monoclonal Antibody with High Affinity and Specificity against Aflatoxins: A Discovery from Rosetta Antibody-Ligand Computational Simulation
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-08-19 , DOI: 10.1021/acs.jcim.4c00736 Changrui Xing 1 , Guanglei Li 1 , Xin Zheng 1 , Peng Li 1 , Jian Yuan 1 , Wenjing Yan 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-08-19 , DOI: 10.1021/acs.jcim.4c00736 Changrui Xing 1 , Guanglei Li 1 , Xin Zheng 1 , Peng Li 1 , Jian Yuan 1 , Wenjing Yan 2
Affiliation
|
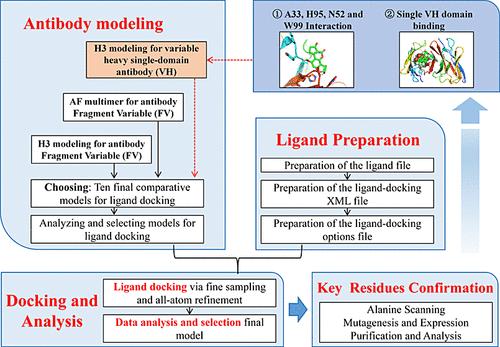
Aflatoxin B1 (AFB1) accumulates in crops, where it poses a threat to human health. To detect AFB1, anti-AFB1 monoclonal antibodies have been developed and are widely used. While the sensitivity and specificity of these antibodies have been extensively studied, information regarding the atomic-level docking of AFB1 (and its derivatives) with these antibodies is limited. Such information is crucial for understanding the key interactions that are required for high affinity and specificity in aflatoxin binding. First, a 3D comparative model of anti-AFB1 antibody (Ab-4B5G6) was predicted from the sequence using RosettaAntibody. We then utilized RosettaLigand to dock AFB1 onto ten homology models, producing a total of 10,000 binding modes. Interestingly, the best-scoring mode predicted strong interactions involving four sites within the heavy chain: ALA33, ASN52, HIS95, and TRP99. Importantly, these strong binding interactions exclusively involve the variable domain of the heavy chain. The best-scoring mode with AFB1 was also obtained through AF multimer combined with RosettaLigand, and two interactions at TRP and HIS were consistent with those found by Rosetta antibody-ligand computational simulation. The role of tryptophan in π interactions in antibodies was confirmed through mutation experiments, and the resulting mutant (W99A) exhibited a >1000-fold reduction in binding affinity for AFB1 and analogs, indicating the effect of tryptophan on the stability of CDR-H3 region. Additionally, we evaluated the binding of two glycolic acid-derived molecular derivatives (with impaired hydrogen bonding potential), and these derivatives (AFB2-GA and AFG2-GA) demonstrated a very weak binding affinity for Ab-4B5G6. The heavy chain was successfully isolated, and its sensitivity and specificity were consistent with those of the intact antibody. The homology models of variable heavy (VH) single-domain antibodies were established by RosettaAntibody, and the docking analysis revealed the same residues, including Ala, His, and Trp. Compared to the potential binding mode of fragment variable (FV) region, the results from a model of VH indicated that there are seven models involved in hydrophobic interaction with TYR32, which is usually referred to as polar amino acid and has both hydrophobic and hydrophilic features depending on the circumstances. Our work encompasses the entire process of Rosetta antibody-ligand computational simulation, highlighting the significance of variable heavy domain structural design in enhancing molecular interactions.
中文翻译:

对黄曲霉毒素具有高亲和力和特异性的新型单克隆抗体的表征:来自 Rosetta 抗体-配体计算模拟的发现
黄曲霉毒素 B1 (AFB 1 ) 在农作物中积累,对人类健康构成威胁。为了检测AFB 1 ,抗AFB 1单克隆抗体已被开发并被广泛使用。虽然这些抗体的敏感性和特异性已得到广泛研究,但有关 AFB 1 (及其衍生物)与这些抗体的原子水平对接的信息有限。这些信息对于理解黄曲霉毒素结合的高亲和力和特异性所需的关键相互作用至关重要。首先,使用 RosettaAntibody 根据序列预测抗 AFB 1抗体 (Ab-4B5G6) 的 3D 比较模型。然后,我们利用 RosettaLigand 将 AFB 1对接到 10 个同源模型上,总共产生 10,000 种结合模式。有趣的是,最佳评分模式预测了涉及重链内四个位点的强相互作用:ALA33、ASN52、HIS95 和 TRP99。重要的是,这些强结合相互作用专门涉及重链的可变域。通过AF多聚体与RosettaLigand结合也获得了AFB 1的最佳评分模式,并且TRP和HIS的两个相互作用与Rosetta抗体-配体计算模拟发现的结果一致。通过突变实验证实了色氨酸在抗体π相互作用中的作用,所得突变体(W99A)对AFB 1和类似物的结合亲和力降低了>1000倍,表明色氨酸对CDR-H3稳定性的影响地区。 此外,我们评估了两种乙醇酸衍生的分子衍生物(氢键电位受损)的结合,这些衍生物(AFB 2 -GA 和 AFG 2 -GA)表现出对 Ab-4B5G6 的结合亲和力非常弱。成功分离出重链,其敏感性和特异性与完整抗体一致。 RosettaAntibody建立了可变重链(VH)单域抗体的同源模型,对接分析显示出相同的残基,包括Ala、His和Trp。与片段可变区(FV)区的潜在结合模式相比,VH模型的结果表明,有七种模型涉及与TYR32的疏水相互作用,TYR32通常被称为极性氨基酸,具有疏水性和亲水性特征视具体情况而定。我们的工作涵盖了 Rosetta 抗体-配体计算模拟的整个过程,强调了可变重域结构设计在增强分子相互作用方面的重要性。
更新日期:2024-08-19
中文翻译:

对黄曲霉毒素具有高亲和力和特异性的新型单克隆抗体的表征:来自 Rosetta 抗体-配体计算模拟的发现
黄曲霉毒素 B1 (AFB 1 ) 在农作物中积累,对人类健康构成威胁。为了检测AFB 1 ,抗AFB 1单克隆抗体已被开发并被广泛使用。虽然这些抗体的敏感性和特异性已得到广泛研究,但有关 AFB 1 (及其衍生物)与这些抗体的原子水平对接的信息有限。这些信息对于理解黄曲霉毒素结合的高亲和力和特异性所需的关键相互作用至关重要。首先,使用 RosettaAntibody 根据序列预测抗 AFB 1抗体 (Ab-4B5G6) 的 3D 比较模型。然后,我们利用 RosettaLigand 将 AFB 1对接到 10 个同源模型上,总共产生 10,000 种结合模式。有趣的是,最佳评分模式预测了涉及重链内四个位点的强相互作用:ALA33、ASN52、HIS95 和 TRP99。重要的是,这些强结合相互作用专门涉及重链的可变域。通过AF多聚体与RosettaLigand结合也获得了AFB 1的最佳评分模式,并且TRP和HIS的两个相互作用与Rosetta抗体-配体计算模拟发现的结果一致。通过突变实验证实了色氨酸在抗体π相互作用中的作用,所得突变体(W99A)对AFB 1和类似物的结合亲和力降低了>1000倍,表明色氨酸对CDR-H3稳定性的影响地区。 此外,我们评估了两种乙醇酸衍生的分子衍生物(氢键电位受损)的结合,这些衍生物(AFB 2 -GA 和 AFG 2 -GA)表现出对 Ab-4B5G6 的结合亲和力非常弱。成功分离出重链,其敏感性和特异性与完整抗体一致。 RosettaAntibody建立了可变重链(VH)单域抗体的同源模型,对接分析显示出相同的残基,包括Ala、His和Trp。与片段可变区(FV)区的潜在结合模式相比,VH模型的结果表明,有七种模型涉及与TYR32的疏水相互作用,TYR32通常被称为极性氨基酸,具有疏水性和亲水性特征视具体情况而定。我们的工作涵盖了 Rosetta 抗体-配体计算模拟的整个过程,强调了可变重域结构设计在增强分子相互作用方面的重要性。































 京公网安备 11010802027423号
京公网安备 11010802027423号