当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ribosome Tunnel Environment Drives the Formation of α-Helix during Cotranslational Folding
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-08-16 , DOI: 10.1021/acs.jcim.4c00901 Takunori Yasuda 1 , Rikuri Morita 2 , Yasuteru Shigeta 2 , Ryuhei Harada 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-08-16 , DOI: 10.1021/acs.jcim.4c00901 Takunori Yasuda 1 , Rikuri Morita 2 , Yasuteru Shigeta 2 , Ryuhei Harada 2
Affiliation
|
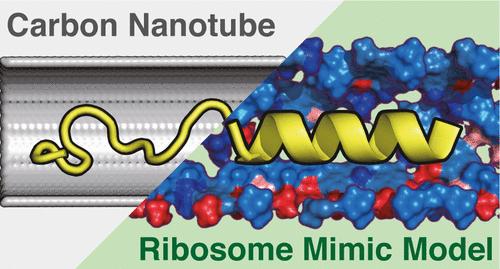
Protein conformations in cells are not solely determined by amino acid sequences; they also depend on cellular environments. For instance, the ribosome tunnel induces its specific α-helix formation during cotranslational folding. Owing to the link between these temporally α-helix and biological functions, the mechanism of α-helix formation inside the ribosome tunnel has been previously explored. Consequently, the conformational restrictions of the tunnel were considered one of the driving forces of α-helix formation. Conversely, the ribosomal tunnel environment, including its chemical properties, appears to influence the α-helix formation. However, a comprehensive analysis of the ribosome tunnel environment’s impact on the α-helix formation has not been conducted yet due to challenges in experimentally controlling it. Therefore, as a new computational approach, we proposed a ribosome environment-mimicking model (REMM) based on the radius and components of the experimentally determined ribosome tunnel structures. Using REMM, we assessed the impact of the ribosome tunnel environment on α-helix formation. Herein, we employed carbon nanotubes (CNT) as a reference model alongside REMM because CNT reproduce conformational restrictions rather than the ribosome tunnel environment. Quantitatively, the ability to reproduce the α-helix of nascent peptides in the experimental structure was compared between the CNT and REMM using enhanced all-atom molecular dynamics simulations. Consequently, the REMM more accurately reproduced the α-helix of the nascent peptides than the CNT, highlighting the significance of the ribosome tunnel environment in α-helix formation. Additionally, we analyzed the properties of the peptide inside each model to reveal the mechanism of ribosome tunnel-specific α-helix formation. Consequently, we revealed that the chemical diversities of the tunnel are essential for the formation of backbone-to-backbone hydrogen bonds in the peptides. In conclusion, the ribosome tunnel environment, with the diverse chemical properties, drives its specific α-helix formation. By proposing REMM, we newly provide the technical basis for investigating the protein conformations in various cellular environments.
中文翻译:

核糖体隧道环境驱动共翻译折叠过程中 α-螺旋的形成
细胞中的蛋白质构象不仅仅由氨基酸序列决定;它们还依赖于蜂窝环境。例如,核糖体隧道在共翻译折叠过程中诱导其特异性 α 螺旋形成。由于这些时间α螺旋与生物学功能之间的联系,以前已经探索了核糖体隧道内 α 螺旋形成的机制。因此,隧道的构象限制被认为是α螺旋形成的驱动力之一。相反,核糖体隧道环境(包括其化学性质)似乎会影响 α 螺旋的形成。然而,由于实验控制核糖体隧道环境的挑战,尚未对核糖体隧道环境对 α-螺旋形成的影响进行全面分析。因此,作为一种新的计算方法,我们提出了一种基于实验确定的核糖体隧道结构的半径和组成部分的核糖体环境模拟模型 (REMM)。使用 REMM,我们评估了核糖体隧道环境对 α 螺旋形成的影响。在此,我们将碳纳米管 (CNT) 与 REMM 一起用作参考模型,因为 CNT 再现构象限制而不是核糖体隧道环境。定量地上,使用增强的全原子分子动力学模拟,比较了 CNT 和 REMM 在实验结构中再现新生肽的 α-螺旋的能力。因此,REMM 比 CNT 更准确地再现了新生肽的 α-螺旋,突出了核糖体隧道环境在 α-螺旋形成中的重要性。 此外,我们分析了每个模型内肽的特性,以揭示核糖体隧道特异性 α 螺旋形成的机制。因此,我们揭示了隧道的化学多样性对于肽中主链-主链氢键的形成至关重要。总之,核糖体隧道环境具有不同的化学性质,驱动其特异性α螺旋的形成。通过提出 REMM,我们为研究各种细胞环境中的蛋白质构象提供了新的技术基础。
更新日期:2024-08-16
中文翻译:

核糖体隧道环境驱动共翻译折叠过程中 α-螺旋的形成
细胞中的蛋白质构象不仅仅由氨基酸序列决定;它们还依赖于蜂窝环境。例如,核糖体隧道在共翻译折叠过程中诱导其特异性 α 螺旋形成。由于这些时间α螺旋与生物学功能之间的联系,以前已经探索了核糖体隧道内 α 螺旋形成的机制。因此,隧道的构象限制被认为是α螺旋形成的驱动力之一。相反,核糖体隧道环境(包括其化学性质)似乎会影响 α 螺旋的形成。然而,由于实验控制核糖体隧道环境的挑战,尚未对核糖体隧道环境对 α-螺旋形成的影响进行全面分析。因此,作为一种新的计算方法,我们提出了一种基于实验确定的核糖体隧道结构的半径和组成部分的核糖体环境模拟模型 (REMM)。使用 REMM,我们评估了核糖体隧道环境对 α 螺旋形成的影响。在此,我们将碳纳米管 (CNT) 与 REMM 一起用作参考模型,因为 CNT 再现构象限制而不是核糖体隧道环境。定量地上,使用增强的全原子分子动力学模拟,比较了 CNT 和 REMM 在实验结构中再现新生肽的 α-螺旋的能力。因此,REMM 比 CNT 更准确地再现了新生肽的 α-螺旋,突出了核糖体隧道环境在 α-螺旋形成中的重要性。 此外,我们分析了每个模型内肽的特性,以揭示核糖体隧道特异性 α 螺旋形成的机制。因此,我们揭示了隧道的化学多样性对于肽中主链-主链氢键的形成至关重要。总之,核糖体隧道环境具有不同的化学性质,驱动其特异性α螺旋的形成。通过提出 REMM,我们为研究各种细胞环境中的蛋白质构象提供了新的技术基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号