当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
GDF11 protects against mitochondrial-dysfunction-dependent NLRP3 inflammasome activation to attenuate osteoarthritis
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-08-03 , DOI: 10.1016/j.jare.2024.08.001 Pengfei Zhang 1 , Haoxin Zhai 1 , Shuai Zhang 2 , Xiaojie Ma 3 , Ao Gong 4 , Zhaoning Xu 5 , Wei Zhao 6 , Hui Song 6 , Shufeng Li 7 , Tengfei Zheng 8 , Zhendong Ying 9 , Lei Cheng 2 , Yunpeng Zhao 2 , Lei Zhang 10
中文翻译:

GDF11 可防止线粒体功能障碍依赖性 NLRP3 炎性小体激活以减轻骨关节炎
骨关节炎 (OA) 是世界范围内高度普遍的退行性疾病,肿瘤坏死因子 (TNF-α) 与其发展密切相关。生长分化因子 11 (GDF11) 已在某些组织中显示出抗损伤和抗衰老能力;然而,它在 OA 中的监管作用仍不清楚,需要进一步调查。
确定 GDF11 是否可以减轻骨关节炎。探讨 GDF11 缓解骨关节炎的潜在机制。
在这项研究中,我们培养和刺激有或没有 TNF-α的小鼠原代软骨细胞,通过微阵列分析产生的损伤表型。此外,我们采用 GDF11 条件性敲除小鼠 OA 模型来检验 GDF11 与 OA 之间的关系。为了研究 GDF11 功能的靶点,我们利用 NLRP3 敲除小鼠及其抑制剂来验证 NLRP3 炎性小体的潜在参与。
我们的体外实验表明,GDF11 的内源性过表达显着抑制 TNF α诱导的软骨细胞软骨基质降解和炎症表达。此外,GDF11 的缺失导致 NLRP3 炎性小体激活、炎症和代谢功能障碍。在体内手术诱导的小鼠模型中,关节内施用重组人 GDF11 减轻了 OA 发病机制,而 GDF11 条件性敲除逆转了这种作用。此外,NLRP3 敲除 DMM 小鼠模型的发现显示,GDF11 通过抑制 NLRP3 发挥其保护作用。
这些发现证明了 GDF11 能够通过预防线粒体功能障碍和抑制 NLRP3 炎性小体激活来抑制 TNF α诱导的炎症和软骨变性,表明其作为骨关节炎治疗药物的潜力。
更新日期:2024-08-03
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-08-03 , DOI: 10.1016/j.jare.2024.08.001 Pengfei Zhang 1 , Haoxin Zhai 1 , Shuai Zhang 2 , Xiaojie Ma 3 , Ao Gong 4 , Zhaoning Xu 5 , Wei Zhao 6 , Hui Song 6 , Shufeng Li 7 , Tengfei Zheng 8 , Zhendong Ying 9 , Lei Cheng 2 , Yunpeng Zhao 2 , Lei Zhang 10
Affiliation
|
Introduction
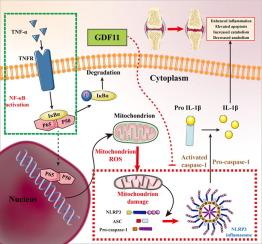
Osteoarthritis (OA) is a highly prevalent degenerative disease worldwide, and tumor necrosis factor (TNF-α) is closely associated with its development. Growth differentiation factor 11 (GDF11) has demonstrated anti-injury and anti-aging abilities in certain tissues; however, its regulatory role in OA remains unclear and requires further investigation.Objectives
To identify whether GDF11 can attenuate osteoarthritis. To exploring the the potential mechanism of GDF11 in alleviating osteoarthritis.Methods
In this study, we cultured and stimulated mouse primary chondrocytes with or without TNF-α, analyzing the resulting damage phenotype through microarray analysis. Additionally, we employed GDF11 conditional knockout mice OA model to examine the relationship between GDF11 and OA. To investigate the target of GDF11′s function, we utilized NLRP3 knockout mice and its inhibitor to verify the potential involvement of the NLRP3 inflammasome.Results
Our in vitro experiments demonstrated that endogenous overexpression of GDF11 significantly inhibited TNF-α-induced cartilage matrix degradation and inflammatory expression in chondrocytes. Furthermore, loss of GDF11 led to NLRP3 inflammasome activation, inflammation, and metabolic dysfunction. In an in vivo surgically induced mouse model, intraarticular administration of recombinant human GDF11 alleviated OA pathogenesis, whereas GDF11 conditional knockout reversed this effect. Additionally, findings from the NLRP3-knockout DMM mouse model revealed that GDF11 exerted its protective effect by inhibiting NLRP3.Conclusion
These findings demonstrate the ability of GDF11 to suppress TNF-α-induced inflammation and cartilage degeneration by preventing mitochondrial dysfunction and inhibiting NLRP3 inflammasome activation, suggesting its potential as a promising therapeutic drug for osteoarthritis.中文翻译:

GDF11 可防止线粒体功能障碍依赖性 NLRP3 炎性小体激活以减轻骨关节炎
介绍
骨关节炎 (OA) 是世界范围内高度普遍的退行性疾病,肿瘤坏死因子 (TNF-α) 与其发展密切相关。生长分化因子 11 (GDF11) 已在某些组织中显示出抗损伤和抗衰老能力;然而,它在 OA 中的监管作用仍不清楚,需要进一步调查。
目标
确定 GDF11 是否可以减轻骨关节炎。探讨 GDF11 缓解骨关节炎的潜在机制。
方法
在这项研究中,我们培养和刺激有或没有 TNF-α的小鼠原代软骨细胞,通过微阵列分析产生的损伤表型。此外,我们采用 GDF11 条件性敲除小鼠 OA 模型来检验 GDF11 与 OA 之间的关系。为了研究 GDF11 功能的靶点,我们利用 NLRP3 敲除小鼠及其抑制剂来验证 NLRP3 炎性小体的潜在参与。
结果
我们的体外实验表明,GDF11 的内源性过表达显着抑制 TNF α诱导的软骨细胞软骨基质降解和炎症表达。此外,GDF11 的缺失导致 NLRP3 炎性小体激活、炎症和代谢功能障碍。在体内手术诱导的小鼠模型中,关节内施用重组人 GDF11 减轻了 OA 发病机制,而 GDF11 条件性敲除逆转了这种作用。此外,NLRP3 敲除 DMM 小鼠模型的发现显示,GDF11 通过抑制 NLRP3 发挥其保护作用。
结论
这些发现证明了 GDF11 能够通过预防线粒体功能障碍和抑制 NLRP3 炎性小体激活来抑制 TNF α诱导的炎症和软骨变性,表明其作为骨关节炎治疗药物的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号