当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ADAM8 deficiency in macrophages promotes cardiac repair after myocardial infarction via ANXA2-mTOR-autophagy pathway
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-08-02 , DOI: 10.1016/j.jare.2024.07.037 Zhenjun Ji 1 , Jiaqi Guo 1 , Rui Zhang 1 , Wenjie Zuo 2 , Yang Xu 1 , Yangyang Qu 1 , Zaixiao Tao 1 , Xinxin Li 1 , Yongjun Li 1 , Yuyu Yao 1 , Genshan Ma 1
中文翻译:

巨噬细胞 ADAM8 缺乏通过 ANXA2-mTOR 自噬通路促进心肌梗死后心脏修复
解整合素和金属蛋白酶 8 (ADAM8) 是巨噬细胞中的关键调节因子,与心血管疾病进展密切相关。
本研究旨在探讨 ADAM8 如何调节心肌梗死 (MI) 后巨噬细胞功能以抑制心脏修复。
使用 CRISPR/Cas9 系统建立巨噬细胞特异性 ADAM8 敲除小鼠 (ADAM8flox/flox, Lyz2-Cre, KO) 和相应的对照小鼠 (ADAM8flox/flox, Flox)。进行骨髓移植,产生巨噬细胞特异性 ADAM8 过表达腺相关病毒 (AAV6-CD68-Adam8)。最后,采用蛋白质组学、RNA 测序和免疫共沉淀/质谱 (COIP/MS) 探讨所涉及的潜在机制。
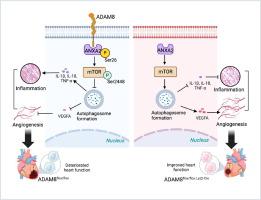
ADAM8 在急性心肌梗死 (AMI) 患者的血浆和 AMI 小鼠的心脏巨噬细胞中高表达。ADAM8 KO 小鼠在 AMI 期间表现出血管生成增强、炎症抑制、心脏纤维化减少和心脏功能改善,这些通过过表达巨噬细胞特异性 ADAM8 和临床抗血管生成生物制剂贝伐珠单抗干预而逆转。骨髓移植实验产生 ADAM8 KO 表型。RNA 测序显示,ADAM8 KO 在骨髓来源的巨噬细胞 (BMDM) 中激活了自噬,这通过 p-mTOR Ser2448/mTOR 、 p62 和 LC3II/I 检测得到证实。自噬失活抑制血管生成因子释放并促进 ADAM8 KO BMDM 的炎症。从机制上讲,ADAM8 可以与 ANXA2 结合并促进 ANXA2 Ser26 位点的磷酸化。ADAM8 KO 阻碍 ANXA2 磷酸化,抑制 mTOR Ser2448 位点磷酸化,并激活自噬,这些通过 ANXA2 磷酸化的激活或失活得到证实。
AMI 后心脏巨噬细胞中 ADAM8 升高。巨噬细胞中的 ADAM8-ANXA2-mTOR-自噬轴负责调节 MI 后的血管生成和炎症。因此,ADAM8 可能是 MI 治疗的新靶点。
更新日期:2024-08-02
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-08-02 , DOI: 10.1016/j.jare.2024.07.037 Zhenjun Ji 1 , Jiaqi Guo 1 , Rui Zhang 1 , Wenjie Zuo 2 , Yang Xu 1 , Yangyang Qu 1 , Zaixiao Tao 1 , Xinxin Li 1 , Yongjun Li 1 , Yuyu Yao 1 , Genshan Ma 1
Affiliation
|
Introduction
A disintegrin and metalloproteinase 8 (ADAM8), a crucial regulator in macrophages, is closely associated with cardiovascular disease progression.Objectives
This study aimed to explore how ADAM8 regulates macrophage function to inhibit cardiac repair after myocardial infarction (MI).Methods
Macrophage-specific ADAM8 knockout mice (ADAM8flox/flox, Lyz2-Cre, KO) and corresponding control mice (ADAM8flox/flox, Flox) were established using the CRISPR/Cas9 system. Bone marrow transplantation was performed, and macrophage-specific ADAM8-overexpressing adeno-associated virus (AAV6-CD68-Adam8) was produced. Finally, proteomics, RNA sequencing, and co-immunoprecipitation/mass spectrometry (COIP/MS) were used to explore the underlying mechanisms involved.Results
ADAM8 was highly expressed in the plasma of patients with acute myocardial infarction (AMI) and in cardiac macrophages derived from AMI mice. ADAM8 KO mice exhibited enhanced angiogenesis, suppressed inflammation, reduced cardiac fibrosis, and improved cardiac function during AMI, which were reversed by overexpressing macrophage-specific ADAM8 and intervention with the clinical anti-angiogenic biologic bevacizumab. Bone marrow transplantation experiments produced ADAM8 KO phenotypes. RNA sequencing showed that autophagy was activated in bone marrow-derived macrophages (BMDMs) with ADAM8 KO, which was confirmed via p-mTOR Ser2448/mTOR, p62, and LC3II/I detection. Autophagy inactivation suppressed angiogenic factor release and promoted inflammation in BMDMs with ADAM8 KO. Mechanistically, ADAM8 could bind to ANXA2 and promote phosphorylation of the ANXA2 Ser26 site. ADAM8 KO impeded ANXA2 phosphorylation, inhibited mTOR Ser2448 site phosphorylation, and activated autophagy, which were demonstrated using the activation or inactivation of ANXA2 phosphorylation.Conclusions
ADAM8 was increased in cardiac macrophages after AMI. The ADAM8-ANXA2-mTOR-autophagy axis in macrophages is responsible for regulating angiogenesis and inflammation following MI. Thus, ADAM8 may be a new target in MI treatment.中文翻译:

巨噬细胞 ADAM8 缺乏通过 ANXA2-mTOR 自噬通路促进心肌梗死后心脏修复
介绍
解整合素和金属蛋白酶 8 (ADAM8) 是巨噬细胞中的关键调节因子,与心血管疾病进展密切相关。
目标
本研究旨在探讨 ADAM8 如何调节心肌梗死 (MI) 后巨噬细胞功能以抑制心脏修复。
方法
使用 CRISPR/Cas9 系统建立巨噬细胞特异性 ADAM8 敲除小鼠 (ADAM8flox/flox, Lyz2-Cre, KO) 和相应的对照小鼠 (ADAM8flox/flox, Flox)。进行骨髓移植,产生巨噬细胞特异性 ADAM8 过表达腺相关病毒 (AAV6-CD68-Adam8)。最后,采用蛋白质组学、RNA 测序和免疫共沉淀/质谱 (COIP/MS) 探讨所涉及的潜在机制。
结果
ADAM8 在急性心肌梗死 (AMI) 患者的血浆和 AMI 小鼠的心脏巨噬细胞中高表达。ADAM8 KO 小鼠在 AMI 期间表现出血管生成增强、炎症抑制、心脏纤维化减少和心脏功能改善,这些通过过表达巨噬细胞特异性 ADAM8 和临床抗血管生成生物制剂贝伐珠单抗干预而逆转。骨髓移植实验产生 ADAM8 KO 表型。RNA 测序显示,ADAM8 KO 在骨髓来源的巨噬细胞 (BMDM) 中激活了自噬,这通过 p-mTOR Ser2448/mTOR 、 p62 和 LC3II/I 检测得到证实。自噬失活抑制血管生成因子释放并促进 ADAM8 KO BMDM 的炎症。从机制上讲,ADAM8 可以与 ANXA2 结合并促进 ANXA2 Ser26 位点的磷酸化。ADAM8 KO 阻碍 ANXA2 磷酸化,抑制 mTOR Ser2448 位点磷酸化,并激活自噬,这些通过 ANXA2 磷酸化的激活或失活得到证实。
结论
AMI 后心脏巨噬细胞中 ADAM8 升高。巨噬细胞中的 ADAM8-ANXA2-mTOR-自噬轴负责调节 MI 后的血管生成和炎症。因此,ADAM8 可能是 MI 治疗的新靶点。































 京公网安备 11010802027423号
京公网安备 11010802027423号