当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Bonding and Physical Trapping of Sulfur in Mesoporous Magnéli Ti4O7 Microspheres for High‐Performance Li–S Battery
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2016-10-31 , DOI: 10.1002/aenm.201601616
Hao Wei 1 , Erwin F. Rodriguez 1 , Adam S. Best 2 , Anthony F. Hollenkamp 3 , Dehong Chen 1 , Rachel A. Caruso 1, 2
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2016-10-31 , DOI: 10.1002/aenm.201601616
Hao Wei 1 , Erwin F. Rodriguez 1 , Adam S. Best 2 , Anthony F. Hollenkamp 3 , Dehong Chen 1 , Rachel A. Caruso 1, 2
Affiliation
|
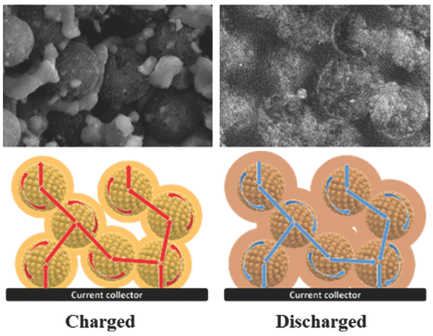
Various host materials have been investigated to address the intrinsic drawbacks of lithium sulfur batteries, such as the low electronic conductivity of sulfur and inevitable decay in capacity during cycling. Besides the widely investigated carbonaceous materials, metal oxides have drawn much attention because they form strong chemical bonds with the soluble lithium polysulfides. Here, mesoporous Magnéli Ti4O7 microspheres are prepared via an in situ carbothermal reduction that exhibit interconnected mesopores (20.4 nm), large pore volume (0.39 cm3 g−1), and high surface area (197.2 m2 g−1). When the sulfur cathode is embedded in a matrix of mesoporous Magnéli Ti4O7 microspheres, it exhibits a superior reversible capacity of 1317.6 mA h g−1 at moderate current (C/10) and a low decay in capacity of 12% after 400 cycles at C/5. Strong chemical bonding of the lithium polysulfides to Ti4O7, as well as effective physical trapping in the mesopores and voids in the matrix are considered responsible for the improved electrochemical performance. A mechanism of the physical and chemical interactions between mesoporous Magnéli Ti4O7 microspheres and sulfur is proposed based on systematic investigations.
中文翻译:

高性能Li–S电池中孔MagnéliTi4O7微球中硫的化学键合和物理捕集
为了解决锂硫电池的固有缺点,已研究了各种主体材料,例如硫的低电子电导率和循环过程中不可避免的容量衰减。除了被广泛研究的碳质材料外,金属氧化物还引起了人们的广泛关注,因为它们与可溶性多硫化锂形成了牢固的化学键。在此,通过原位碳热还原制备了介孔的MagnéliTi 4 O 7微球,该微球表现出相互连通的中孔(20.4 nm),大孔体积(0.39 cm 3 g -1)和高表面积(197.2 m 2 g -1) 。当硫阴极嵌入介孔的MagnéliTi 4基质中时在7个微球中,它在中等电流(C / 10)下表现出1317.6 mA hg -1的优异可逆容量,在C / 5下经过400次循环后其容量衰减低至12%。认为多硫化锂与Ti 4 O 7的牢固化学键结合以及在介孔和基质中的空隙中的有效物理捕集是改善电化学性能的原因。在系统研究的基础上,提出了介孔的MagnéliTi 4 O 7微球与硫之间的物理和化学相互作用的机理。
更新日期:2016-10-31
中文翻译:

高性能Li–S电池中孔MagnéliTi4O7微球中硫的化学键合和物理捕集
为了解决锂硫电池的固有缺点,已研究了各种主体材料,例如硫的低电子电导率和循环过程中不可避免的容量衰减。除了被广泛研究的碳质材料外,金属氧化物还引起了人们的广泛关注,因为它们与可溶性多硫化锂形成了牢固的化学键。在此,通过原位碳热还原制备了介孔的MagnéliTi 4 O 7微球,该微球表现出相互连通的中孔(20.4 nm),大孔体积(0.39 cm 3 g -1)和高表面积(197.2 m 2 g -1) 。当硫阴极嵌入介孔的MagnéliTi 4基质中时在7个微球中,它在中等电流(C / 10)下表现出1317.6 mA hg -1的优异可逆容量,在C / 5下经过400次循环后其容量衰减低至12%。认为多硫化锂与Ti 4 O 7的牢固化学键结合以及在介孔和基质中的空隙中的有效物理捕集是改善电化学性能的原因。在系统研究的基础上,提出了介孔的MagnéliTi 4 O 7微球与硫之间的物理和化学相互作用的机理。





































 京公网安备 11010802027423号
京公网安备 11010802027423号