当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Use of a Cyclic α-Alkylidene-β-Diketone as a Cleavable Linker Strategy for Antibody-Drug Conjugates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-08-15 , DOI: 10.1021/jacs.4c04567 Juliana T W Tong 1, 2 , Makhdoom Sarwar 2, 3 , Marzieh Ahangarpour 1, 2 , Paul A Hume 3, 4, 5 , Geoffrey M Williams 1 , Margaret A Brimble 1, 2 , Iman Kavianinia 2, 6
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-08-15 , DOI: 10.1021/jacs.4c04567 Juliana T W Tong 1, 2 , Makhdoom Sarwar 2, 3 , Marzieh Ahangarpour 1, 2 , Paul A Hume 3, 4, 5 , Geoffrey M Williams 1 , Margaret A Brimble 1, 2 , Iman Kavianinia 2, 6
Affiliation
|
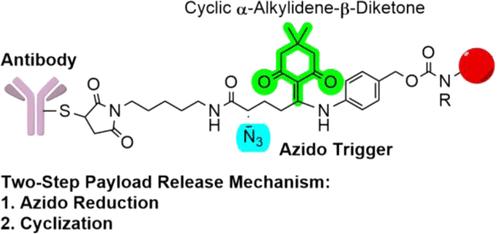
In the fast-evolving landscape of targeted cancer therapies, the revolutionary class of biotherapeutics known as antibody-drug conjugates (ADCs) are taking center stage. Most clinically approved ADCs utilize cleavable linkers to temporarily attach potent cytotoxic payloads to antibodies, allowing selective payload release under tumor-specific conditions. In this study, we explored the utilization of 1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethyl (Dde), a cyclic β-diketone featuring an active alkylidene group, to develop a novel chemically labile linker. This linker was designed to exploit the difference in reduction potential between the intracellular compartment and plasma. Upon reduction of an azido trigger strategically installed neighboring the cyclic β-diketone, the resulting nucleophilic primary amine reacts with the alkylidene group facilitated by a favorable ring closure reaction in accordance with Baldwin’s rules. Consequently, this reaction enables the simultaneous release of the attached cytotoxic payload. The therapeutic utility of this novel linker strategy was demonstrated by separate conjugation of the linker to two epidermal growth factor receptor (EGFR)-targeting ligands to afford a peptide-drug conjugate and an ADC. This work comprises a significant contribution to the bioconjugation field by introducing the alkylidene cyclic β-diketone as a tunable scaffold used for the temporary conjugation of therapeutic agents to peptides and proteins.
中文翻译:

使用环状 α-亚烷基-β-二酮作为抗体-药物缀合物的可切割接头策略
在快速发展的靶向癌症治疗领域,被称为抗体药物偶联物 (ADC) 的革命性生物治疗药物正在占据中心舞台。大多数临床批准的 ADC 利用可裂解连接体将有效的细胞毒性有效负载暂时连接到抗体上,从而允许在肿瘤特定条件下选择性释放有效负载。在本研究中,我们探索利用 1-(4,4-二甲基-2,6-二氧亚环己基)乙基 (Dde)(一种具有活性亚烷基的环状 β-二酮)来开发一种新型化学不稳定连接体。该接头旨在利用细胞内室和血浆之间还原电位的差异。当策略性地安装在环状 β-二酮附近的叠氮触发物被还原时,根据鲍德温规则,所产生的亲核伯胺与亚烷基发生反应,通过有利的闭环反应来促进。因此,该反应能够同时释放附着的细胞毒性有效负载。通过将接头与两个表皮生长因子受体 (EGFR) 靶向配体单独缀合以提供肽-药物缀合物和 ADC,证明了这种新型接头策略的治疗效用。这项工作通过引入亚烷基环β-二酮作为可调节支架,用于治疗剂与肽和蛋白质的临时缀合,对生物缀合领域做出了重大贡献。
更新日期:2024-08-15
中文翻译:

使用环状 α-亚烷基-β-二酮作为抗体-药物缀合物的可切割接头策略
在快速发展的靶向癌症治疗领域,被称为抗体药物偶联物 (ADC) 的革命性生物治疗药物正在占据中心舞台。大多数临床批准的 ADC 利用可裂解连接体将有效的细胞毒性有效负载暂时连接到抗体上,从而允许在肿瘤特定条件下选择性释放有效负载。在本研究中,我们探索利用 1-(4,4-二甲基-2,6-二氧亚环己基)乙基 (Dde)(一种具有活性亚烷基的环状 β-二酮)来开发一种新型化学不稳定连接体。该接头旨在利用细胞内室和血浆之间还原电位的差异。当策略性地安装在环状 β-二酮附近的叠氮触发物被还原时,根据鲍德温规则,所产生的亲核伯胺与亚烷基发生反应,通过有利的闭环反应来促进。因此,该反应能够同时释放附着的细胞毒性有效负载。通过将接头与两个表皮生长因子受体 (EGFR) 靶向配体单独缀合以提供肽-药物缀合物和 ADC,证明了这种新型接头策略的治疗效用。这项工作通过引入亚烷基环β-二酮作为可调节支架,用于治疗剂与肽和蛋白质的临时缀合,对生物缀合领域做出了重大贡献。































 京公网安备 11010802027423号
京公网安备 11010802027423号